Expired Mupirocin Risks: What Every Patient Should Know
- Colin Hurd
- 19 October 2025
- 8 Comments
Mupirocin Expiration Safety Checker
Check Your Mupirocin Safety
Enter your mupirocin tube information below to determine if it's still safe to use.
Ever grabbed that tiny tube of ointment from your medicine cabinet and wondered if it’s still good? When it comes to expired mupirocin, the answer matters - using it past its date can turn a simple skin infection into a bigger problem.
What is mupirocin?
Mupirocin is a topical antibiotic ointment used to treat skin infections caused by certain bacteria, such as Staphylococcus aureus and Streptococcus pyogenes. It works by blocking bacterial protein synthesis, which stops the microbes from growing. Because it’s applied directly to the skin, it’s especially handy for things like impetigo, minor cuts, and infected eczema.
Why does expiration matter?
Every medication has a shelf life - a period during which the manufacturer guarantees full potency and safety. After that date, the chemical composition can start to break down. For mupirocin, degradation can mean:
- Reduced antimicrobial potency, so the ointment may no longer kill the targeted bugs.
- Formation of breakdown products that could irritate the skin.
- A higher chance that surviving bacteria develop resistance.
In short, an out‑of‑date tube may be less effective and potentially harmful.
Real risks of using expired mupirocin
Let’s break down the three biggest dangers:
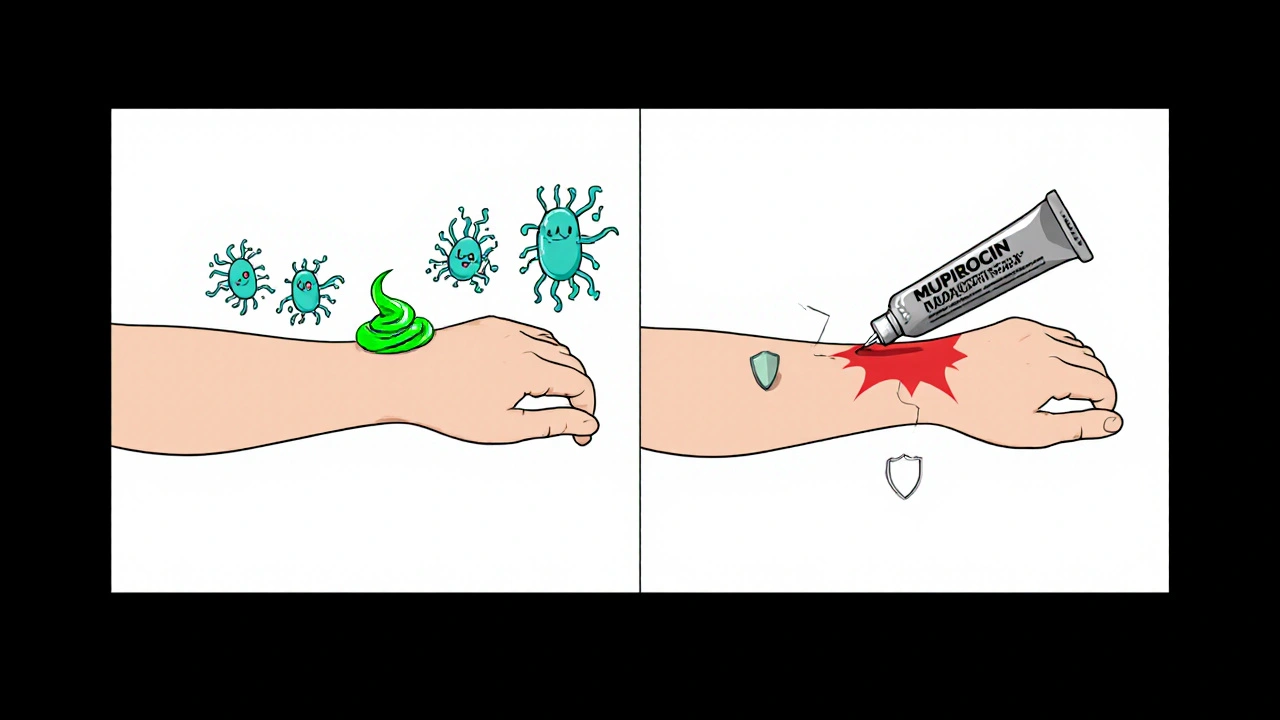
- Ineffective treatment: If the ointment can’t suppress Staphylococcus aureus or Streptococcus pyogenes, the infection can spread, leading to larger lesions, fever, or even systemic infection.
- Resistance development: Sub‑therapeutic levels act like a weak antibiotic pressure. Bacteria such as methicillin‑resistant MRSA may survive and mutate, making future infections harder to treat.
- Skin irritation or allergic reaction: Degraded compounds can change pH or create irritants, causing redness, itching, or contact dermatitis.
These risks aren’t just theoretical - case reports from dermatology clinics have documented treatment failures linked to expired topical antibiotics, especially when patients assume “a little went bad” won’t matter.
How to spot an expired tube
Pharmacies are required to print a “use by” or “expiration” date on the packaging. Here’s a quick checklist:
- Locate the date on the tube cap, label, or carton.
- Check for any “expired” stamp that a pharmacist might add when the product is returned.
- Inspect the ointment’s texture - a thickened, discolored, or foul‑smelling product often signals degradation.
- Consider storage conditions. Mupirocin should stay at room temperature, away from direct sunlight. Heat or moisture can accelerate loss of potency.
If any of these red flags appear, it’s safer to toss the tube.
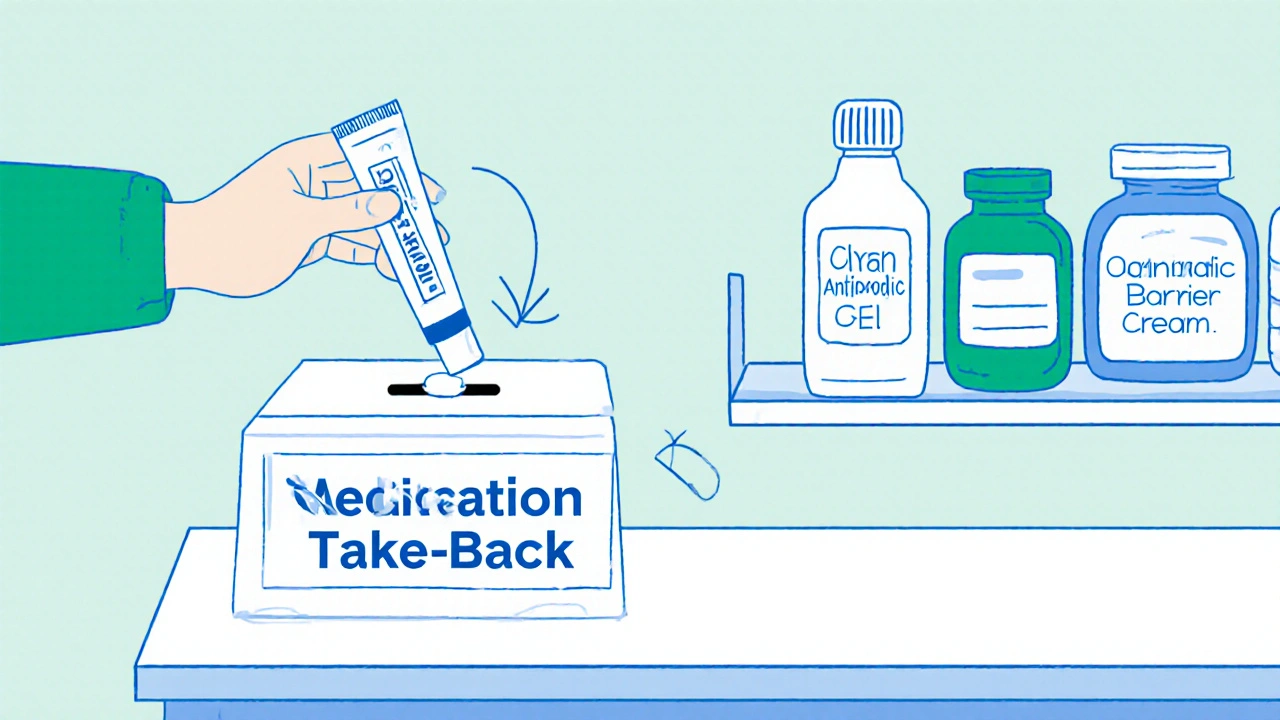
What to do with expired mupirocin
Don’t just toss it in the trash. Many pharmacy chains and local councils run medication take‑back programs. In Australia, you can drop expired medicines at any pharmacy participating in the National Medicines Disposal Program.
While you’re waiting for a replacement, consider these alternatives:
- Clindamycin topical gel - effective against many of the same bacteria.
- Oral antibiotics prescribed by a doctor if the infection is extensive.
- Proper wound care - clean the area with mild soap, keep it dry, and apply a non‑antibiotic barrier cream.
Always consult a pharmacist or doctor before switching treatments.
Comparison: In‑date vs. Expired mupirocin
| Attribute | In‑date (within shelf life) | Expired (past shelf life) |
|---|---|---|
| Potency | ≥ 95% of labeled strength | Often < 70%, variable |
| Bacterial coverage | Effective against S. aureus, MRSA, S. pyogenes | Reduced, gaps in coverage |
| Risk of resistance | Low when used as directed | Higher due to sub‑therapeutic dosing |
| Skin irritation | Rare, mild | Possible, due to breakdown products |
| Regulatory status | Approved for use by FDA/TGA | Considered unsafe; not marketable |
Bottom line: When in doubt, discard
Using an expired tube of mupirocin isn’t a harmless shortcut. The loss of potency, increased resistance risk, and possible skin irritation make it a gamble you don’t need to take. Keep an eye on dates, store your ointments properly, and follow local disposal guidelines.
Can I use a slightly out‑of‑date mupirocin tube?
Even a few months past the printed date can reduce its antibacterial strength. It’s best to replace it to ensure effective treatment.
How long does mupirocin stay effective after opening?
Once opened, keep the tube tightly capped and store at room temperature. Most manufacturers recommend using it within 6‑12 months, but always follow the label.
What are the signs of a bacterial skin infection that needs mupirocin?
Redness, swelling, pus‑filled lesions, or a painful crusty rash (often called impetigo) are typical. If you notice fever or spreading redness, seek medical help.
Is it safe to share my mupirocin with a family member?
Only use your own prescription. Different infections may need different dosages or even different antibiotics. Sharing can lead to misuse and resistance.
Where can I safely dispose of expired mupirocin in Perth?
Take it to any participating pharmacy in the WA Medication Take‑Back program, or drop it off at a local council collection point. Do not flush it down the toilet.



Comments
Latasha Becker
The pharmacokinetic stability of mupirocin is contingent upon its esterified lipid matrix, which undergoes hydrolytic degradation post‑expiration.
Empirical studies have demonstrated a quantifiable decline in minimum inhibitory concentration (MIC) values after the labeled shelf‑life.
Consequently, the bacteriostatic threshold is compromised, permitting residual microbial proliferation.
Moreover, oxidative by‑products such as mupirocinic acid can act as haptenic determinants, precipitating contact dermatitis.
This dermatologic sequela is not merely anecdotal but corroborated by peer‑reviewed case series in dermatology journals.
In addition, sub‑therapeutic dosing fosters selective pressure that accelerates the emergence of methicillin‑resistant Staphylococcus aureus (MRSA) clones.
The regulatory framework of the FDA mandates a rigorous post‑marketing surveillance to avert such iatrogenic resistance.
Patients who self‑administer expired preparations inadvertently contravene these safety protocols.
The degradation kinetics follow a first‑order reaction, with a half‑life approximating 9‑12 months under ambient conditions.
Storage variables, notably temperature excursions exceeding 25 °C, exacerbate this kinetic profile.
Hence, an ostensibly intact tube may harbor diminished potency unbeknownst to the consumer.
The physicochemical alteration also manifests as increased viscosity and chromatic shifts, serving as macroscopic red flags.
Clinical guidelines therefore advocate for the immediate decommissioning of any mupirocin formulation beyond its expiration date.
Disposal should be conducted via authorized medication take‑back programs to mitigate environmental contamination.
Substituting with alternative agents such as clindamycin gel necessitates physician oversight to circumvent cross‑resistance.
Ultimately, adherence to expiration timelines is a non‑negotiable component of antimicrobial stewardship.
October 19, 2025 AT 12:58
parth gajjar
Oh the tragedy of a forgotten tube, its former glory now a silent assassin lurking in the bathroom cabinet! The oily residue whispers promises of cure but delivers only irritation and the bitter taste of wasted hope.
One might think a few months past the date is harmless, yet the molecule itself has begun to decay, shedding its antimicrobial armor.
Imagine the bacteria laughing, thriving on the weakened formula, turning a simple rash into a battlefield.
November 1, 2025 AT 13:26
Madhav Dasari
Hey there, let’s keep it simple – even if that ointment looks fine, the chemistry inside can change faster than you think.
If you’ve got any sign of discoloration or weird smell, toss it and grab a fresh one.
Better safe than sorry, and your skin will thank you for not taking chances.
November 13, 2025 AT 03:13
DHARMENDER BHATHAVAR
Expired mupirocin loses potency quickly; replace it promptly.
November 24, 2025 AT 17:00
Kevin Sheehan
While the concise advice to replace expired ointment is sound, one must also contemplate the ethical dimensions of self‑medication; relying on stale antibiotics without professional guidance borders on reckless individualism, undermining collective health security.
December 6, 2025 AT 06:46
Jay Kay
Don’t use expired mupirocin.
December 17, 2025 AT 20:33
Rakhi Kasana
It’s a straightforward rule – if the tube is past its date, just drop it in the pharmacy’s take‑back bin; no need to overthink it.
December 29, 2025 AT 10:20
jagdish soni
Hey folks, just a friendly heads‑up: those little tubs of mupirocin might look fine, but once they’re past their prime they’re basically just decorative ointment.
If you’re unsure, swing by your local pharmacy’s disposal spot – it’s quick, easy, and keeps the environment happy.
January 10, 2026 AT 00:06