How to Compare Generic Manufacturers and Pill Appearance
- Colin Hurd
- 19 December 2025
- 11 Comments
When you pick up your prescription and see that your pill looks completely different from last time, it’s normal to feel confused. Maybe it’s a different color, shape, or has strange letters stamped on it. You might wonder: Is this still the same medicine? The answer is almost always yes - but knowing how to check makes all the difference.
Why Generic Pills Look Different
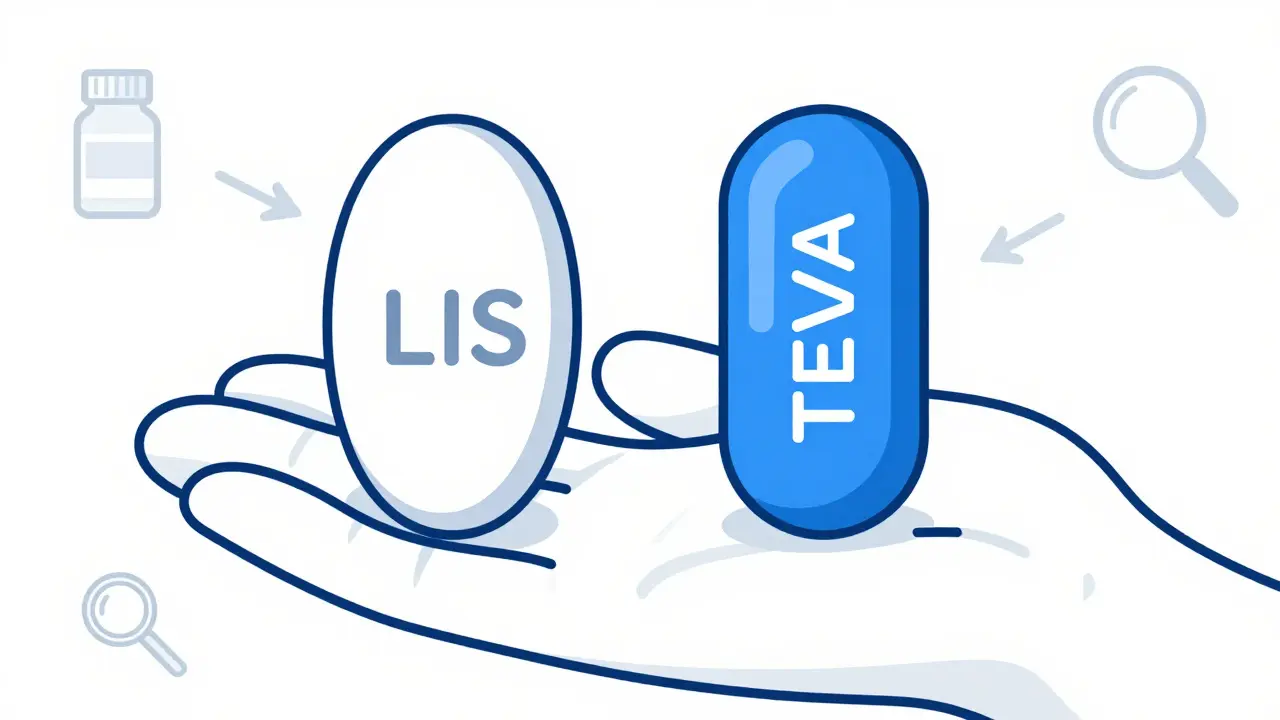
Generic drugs are not copies of brand-name pills in appearance. By law, they can’t be. The U.S. Food and Drug Administration (FDA) requires that generic versions look different from the brand-name drug to avoid trademark infringement. So even if the active ingredient is identical, the color, shape, size, or imprint can change every time a different manufacturer makes it. For example, the brand-name version of lisinopril might be a small white oval tablet with "LIS" stamped on it. But the generic version from Teva could be a blue capsule, while the one from Mylan is a yellow round tablet with "M 12" printed on it. Both contain the exact same amount of lisinopril and work the same way. The difference is purely cosmetic - and legal. This isn’t a trick. It’s a rule. The FDA mandates unique imprints under the 1970 Federal Food, Drug, and Cosmetic Act amendments so pharmacists, doctors, and patients can identify pills accurately. That’s why every prescription pill - brand or generic - has a distinct marking. If two pills look identical, one is likely counterfeit.What Makes Generics the Same - and What Doesn’t Matter
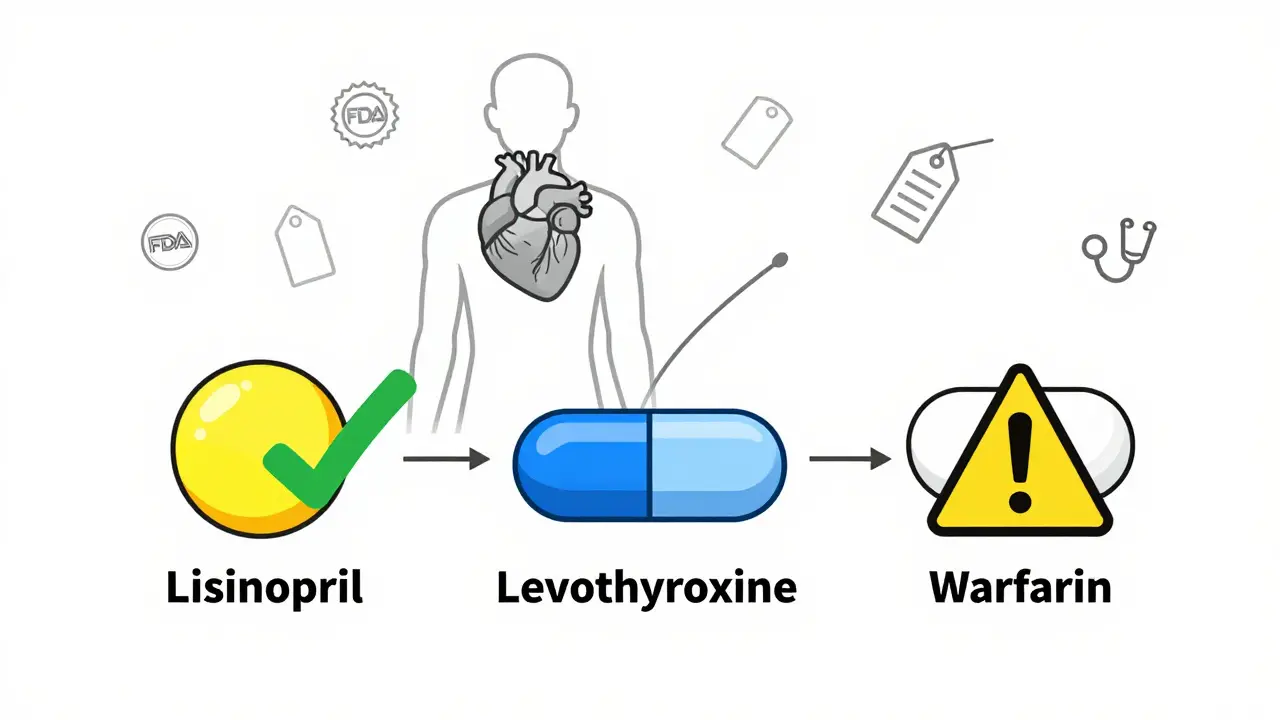
The only things that matter for effectiveness are the active ingredient, strength, dosage form, and how your body absorbs it. That’s called bioequivalence. To get FDA approval, a generic drug must prove it delivers the same amount of medicine into your bloodstream as the brand-name version - within a very tight range. For most drugs, that range is 80% to 125% of the brand-name drug’s absorption rate. That means if the brand delivers 100 units of medicine, the generic must deliver between 80 and 125 units. In real-world terms, studies show the average difference is just 3.5%. That’s less than the variation you’d see between two batches of the same brand-name drug made months apart. But here’s the catch: some drugs are more sensitive. These are called narrow therapeutic index (NTID) drugs - things like warfarin, levothyroxine, phenytoin, and lithium. For these, the acceptable range is tighter: 90% to 111%. Even small changes in absorption can cause problems. That’s why doctors often recommend sticking with the same generic manufacturer for these medications. Inactive ingredients - like dyes, fillers, and binders - don’t affect how the drug works. But they do affect how the pill looks and sometimes how it feels in your mouth. A dye might make a pill blue instead of white. A different filler might make it larger or harder to swallow. These changes are harmless for most people, but if you have allergies (like to certain dyes or gluten), you should always check the inactive ingredients list on the label.How to Identify Your Generic Pill
You don’t need to guess whether your pill is right. There are simple, reliable tools to confirm it. Start with the imprint. Every pill has letters, numbers, or symbols stamped on it. Combine that with the shape and color, and you can look it up. The Drugs.com Pill Identifier is used by over 12 million people each month. Just enter the color, shape, and imprint - and it shows you exactly what the pill is, who makes it, and what it’s used for. Pharmacists use the National Drug Code (NDC) directory. That’s a 10-digit number on the bottle that tells you the manufacturer, drug name, strength, and package size. If you’re unsure, ask your pharmacist to show you the NDC. They can tell you if this is the same drug as last time - even if it looks different. You can also check the FDA’s Drugs@FDA database. Search for your drug by name, and you’ll see every approved version - brand and generic - with their imprint codes and manufacturers listed. It’s free, official, and updated daily.
Who Makes Generic Drugs - And Why It Matters
Not all generic manufacturers are the same. There are big names like Teva, Mylan (now part of Viatris), Sandoz, and Hikma. Then there are smaller companies that make less common generics. Teva is the world’s largest generic manufacturer. They make about 1 in 10 generic prescriptions in the U.S. Mylan and Sandoz are also major players. These companies invest in consistent quality control and have been around for decades. They’re less likely to have supply issues or quality problems. Smaller manufacturers may offer lower prices, but they’re also more likely to have production delays. In 2024, 67% of active drug shortages in the U.S. involved generic medications. That’s not because generics are unsafe - it’s because the market is so competitive, and profit margins are thin. When a factory shuts down for inspection or a supplier runs out of a chemical, the whole supply chain can break. For most people, switching between generic manufacturers is no problem. But for NTID drugs, consistency matters. If you’re on levothyroxine for hypothyroidism, and your pill changes from white to tan, don’t panic - but do talk to your doctor. Your thyroid levels might need checking.What to Do When Your Pill Changes
Here’s a simple step-by-step guide:- Don’t panic. A change in appearance doesn’t mean the drug is wrong.
- Check the imprint. Look for letters or numbers on the pill. Write them down.
- Use Drugs.com or ask your pharmacist. Enter the color, shape, and imprint. Confirm the drug name and manufacturer.
- Compare the NDC number. If the NDC on your bottle changed, that’s why the pill looks different.
- For NTID drugs, call your doctor. If you’re on warfarin, lithium, or levothyroxine, ask if you should stay with the same manufacturer.
- Don’t stop taking it. Unless your doctor says so, keep taking the medication. The active ingredient hasn’t changed.
When to Be Concerned
Most of the time, a new-looking pill is perfectly fine. But there are red flags:- You feel worse after switching - especially with seizure meds, blood thinners, or thyroid drugs.
- The pill looks completely different AND the NDC changed without your pharmacist telling you.
- You’re told the drug is "generic" but it’s not listed in Drugs.com or FDA’s database.
- The pill is cracked, discolored, or smells odd.
Cost vs. Consistency
Generics save money - a lot of it. The average patient saves $265 per month by switching from brand to generic. With 10 or more manufacturers competing, prices can drop 70-80% from the original brand price. But cheaper isn’t always better if you’re on a sensitive medication. If you’ve been stable on one generic for years, switching to a cheaper version might not be worth the risk. Talk to your doctor about whether staying with the same manufacturer is better for your health. Some pharmacies offer "dispense as written" (DAW-1) options. That means they won’t substitute your generic unless you or your doctor says yes. Ask if this is available - it’s a simple way to avoid surprises.What Experts Say
The FDA, American Medical Association, and European Medicines Agency all agree: generics are just as safe and effective as brand-name drugs for nearly everyone. A 2021 EMA review of over 9 million patients found no meaningful difference in outcomes between branded and generic heart medications. Dr. Aaron Kesselheim from Harvard Medical School says: "For most conditions, generics work just as well. But for drugs with a narrow therapeutic index, switching manufacturers should be done carefully - and with monitoring." The bottom line? Don’t fear the change in color or shape. Fear the lack of information. Know how to check your pill. Know your NDC. Know your manufacturer. That’s how you stay in control.Why does my generic pill look different from last time?
Generic pills must look different from brand-name drugs by law to avoid trademark issues. Even if the active ingredient is identical, different manufacturers use different colors, shapes, sizes, and imprints. This is normal and doesn’t affect how the drug works. Always check the imprint and NDC code to confirm it’s the same medication.
Are generic drugs as effective as brand-name drugs?
Yes, for 99.9% of cases. The FDA requires generics to prove they deliver the same amount of medicine into your bloodstream as the brand-name version, within a strict range (80-125% for most drugs). Studies show the average difference is only 3.5%. For most conditions, including high blood pressure, diabetes, and depression, generics work just as well.
Should I stick with the same generic manufacturer?
For most medications, no - switching is safe. But for narrow therapeutic index drugs like warfarin, levothyroxine, or phenytoin, consistency matters. Small changes in absorption can cause side effects or reduced effectiveness. If you’re on one of these, ask your doctor to recommend staying with the same manufacturer. You can request "dispense as written" at your pharmacy.
How can I tell if my pill is the right one?
Use the imprint, color, and shape to look it up on Drugs.com’s Pill Identifier. You can also check the National Drug Code (NDC) on your prescription bottle - it uniquely identifies the manufacturer and product. If you’re unsure, ask your pharmacist to verify it using the FDA’s Drugs@FDA database.
Can generic drugs cause side effects that brand-name drugs don’t?
Rarely. Side effects usually come from the active ingredient, which is the same in both. But inactive ingredients like dyes or fillers can cause allergic reactions in some people. If you notice new symptoms after switching - like a rash, stomach upset, or unusual fatigue - talk to your doctor. Check the label for ingredients you’re sensitive to.
Why are generic drugs so much cheaper?
Generic manufacturers don’t pay for expensive clinical trials or marketing campaigns. They only need to prove bioequivalence to the brand-name drug, which costs far less. Once multiple companies make the same generic, competition drives prices down. With 10 or more manufacturers, prices can drop 70-80% from the original brand price.
What should I do if I think my generic isn’t working?
Don’t stop taking it. First, confirm the pill is correct using the imprint and NDC code. If it’s the right drug, talk to your doctor. For sensitive medications like thyroid or seizure drugs, your dosage might need adjusting after a switch. Blood tests can check if your levels are stable. Never assume the drug is ineffective - it’s usually just a perception issue.
Are there any risks with switching between generic manufacturers?
For most people, no. But for narrow therapeutic index drugs, even small changes in absorption can matter. In rare cases, patients have reported side effects after switching manufacturers of drugs like lamotrigine or levothyroxine. That’s why doctors often recommend staying with the same generic for these medications. Always report any new symptoms after a switch.



Comments
Aboobakar Muhammedali
Man i just got my lisinopril and it was blue this time not white like before i thought i got something wrong
looked it up on drugs.com and it was teva's generic same dose same everything
relieved but also kinda amazed how much we worry over color and shape when the medicine inside is exactly the same
December 20, 2025 AT 20:52
Nicole Rutherford
Oh please like this is some groundbreaking revelation
everybody knows generics look different
the real issue is pharmacies switching manufacturers without telling you and then acting like it's your fault for noticing
and don't even get me started on how they charge you extra for "dispense as written" like it's a luxury service
December 20, 2025 AT 23:14
Dominic Suyo
This whole system is a corporate circus
brand-name pharma spends billions on marketing then sells out to generics who are forced to make pills look different so they can't be confused with the very thing they're replacing
the FDA's "imprint rule" is just legal theater
it's not about safety it's about trademark entropy
and don't get me started on how the same pill made in India looks different than the one made in Germany even though both are "Teva"
we're not protecting patients we're protecting brand equity with a side of bureaucratic theater
December 21, 2025 AT 05:15
Hussien SLeiman
Let me break this down for you people who think this is just about color and shape
the real issue isn't the pill's appearance it's the systemic erosion of pharmaceutical consistency
when you're on a narrow therapeutic index drug like levothyroxine your body adapts to a specific formulation over time
even a 3.5% variation in bioavailability can throw your entire endocrine system into chaos
and yet the pharmacy system treats this like a vending machine where you just grab whatever's cheapest
the fact that doctors don't routinely order "dispense as written" for NTID drugs is a scandal
and don't even mention how the FDA's approval process for generics doesn't require long-term outcome studies only bioequivalence
so we're trusting a 12-week pharmacokinetic study to replace decades of clinical experience
and then we wonder why people report feeling "off" after a switch
it's not placebo it's pharmacological drift
and the system is designed to ignore it because profit margins are thin and accountability is nonexistent
December 22, 2025 AT 05:34
Chris Clark
Actually the FDA's bioequivalence range of 80-125% is statistically robust
the 3.5% average deviation is well within inter-batch variability of brand-name products
and the 90-111% for NTIDs is conservative
the real problem is pharmacists not communicating NDC changes
not the science
the science is solid
the system is broken
but the science? impeccable
December 22, 2025 AT 21:03
Nina Stacey
i had a friend who switched from one generic to another for her thyroid med and she started having panic attacks and weight gain
she went back to the original one and it was like night and day
so yeah maybe the science says its fine
but real people feel real things
and if your body reacts differently to a pill that looks different
then maybe the system needs to listen
not just the lab reports
also i spelled thyroid wrong twice but you know what i mean
December 23, 2025 AT 16:28
anthony funes gomez
What's being overlooked is the macroeconomic asymmetry: generic manufacturers operate on razor-thin margins, which incentivizes consolidation and supply-chain fragility
67% of shortages are generic because there's no profit in redundancy
no buffer stock
no safety margin
only price competition
so when a single factory in Mumbai shuts down for a week
the entire U.S. supply of metformin collapses
and patients get whatever's left
not what's best
the system is optimized for cost
not resilience
not continuity
not patient stability
it's a market failure disguised as a savings program
December 24, 2025 AT 10:00
Dorine Anthony
I just want to say thank you for writing this
I was so scared when my pill changed color last month
thought I was losing my mind
looked it up and it was all good
but I didn't know about the NDC code
my pharmacist showed me how to check it
now I feel way more in control
thanks for the clarity
December 24, 2025 AT 16:14
James Stearns
It is imperative to underscore that the United States Food and Drug Administration has established a rigorous and scientifically validated framework for the approval of generic pharmaceuticals
the requirement for unique imprints is not arbitrary
it is a legally mandated safeguard against counterfeit medications
and the bioequivalence thresholds are derived from decades of peer-reviewed pharmacokinetic research
to suggest otherwise is not merely inaccurate
it is dangerously irresponsible
and frankly
unbecoming of an informed citizenry
December 24, 2025 AT 17:42
Guillaume VanderEst
My mom's on warfarin and they switched her generic last month
she got dizzy
we called the doctor
they checked her INR
it was fine
but she still felt weird
so we asked for the original manufacturer
pharmacy charged us $12 extra
but she hasn't felt dizzy since
so yeah maybe the science says it's fine
but my mom's body says otherwise
and I'll pay the $12 every time
December 26, 2025 AT 02:40
Janelle Moore
Did you know the FDA allows foreign labs to make 80% of our generics?
And that some of them use talc from China that's been linked to cancer?
And that the imprint codes are sometimes just random numbers printed by robots?
And that big pharma owns the biggest generic companies?
And that they change the pill color to trick you into thinking it's new?
It's all a cover-up.
They don't want you to know your medicine is made in a factory with no inspectors.
They just want you to swallow it and shut up.
December 27, 2025 AT 12:02