Authorized Generic Pricing: Why They Cost Less Than Brand Name Drugs
- Colin Hurd
- 4 December 2025
- 12 Comments
Have you ever picked up a prescription and seen two pills that look exactly the same-one with a fancy brand name, another labeled as a generic-and wondered why one costs half as much? If it’s an authorized generic, the answer is simpler than you think: it’s the exact same drug, made in the same factory, under the same rules. But here’s the twist-it’s sold under a different label, and that’s why it’s cheaper.
What Exactly Is an Authorized Generic?
An authorized generic isn’t some knockoff or cut-rate version. It’s the brand-name drug, produced by the original manufacturer, but sold without the brand name on the bottle. Think of it like a car company making the same model but selling it under a different dealership name. The engine, the seats, the paint-all identical. The only difference? The sticker price. The U.S. Food and Drug Administration (FDA) requires these products to meet the same strict standards as the brand-name version. Same active ingredient. Same dosage. Same manufacturing facility. Same quality checks. In fact, authorized generics don’t even need a separate approval process like regular generics do. They’re covered under the original New Drug Application (NDA), which means they skip the Abbreviated New Drug Application (ANDA) route entirely. This isn’t a loophole. It’s a legal strategy built into the Hatch-Waxman Act of 1984. That law gave the first generic company to challenge a patent 180 days of exclusive market access. But brand manufacturers didn’t want to lose all their customers overnight. So they started launching their own authorized generics at the same time.Why Are Authorized Generics Cheaper?
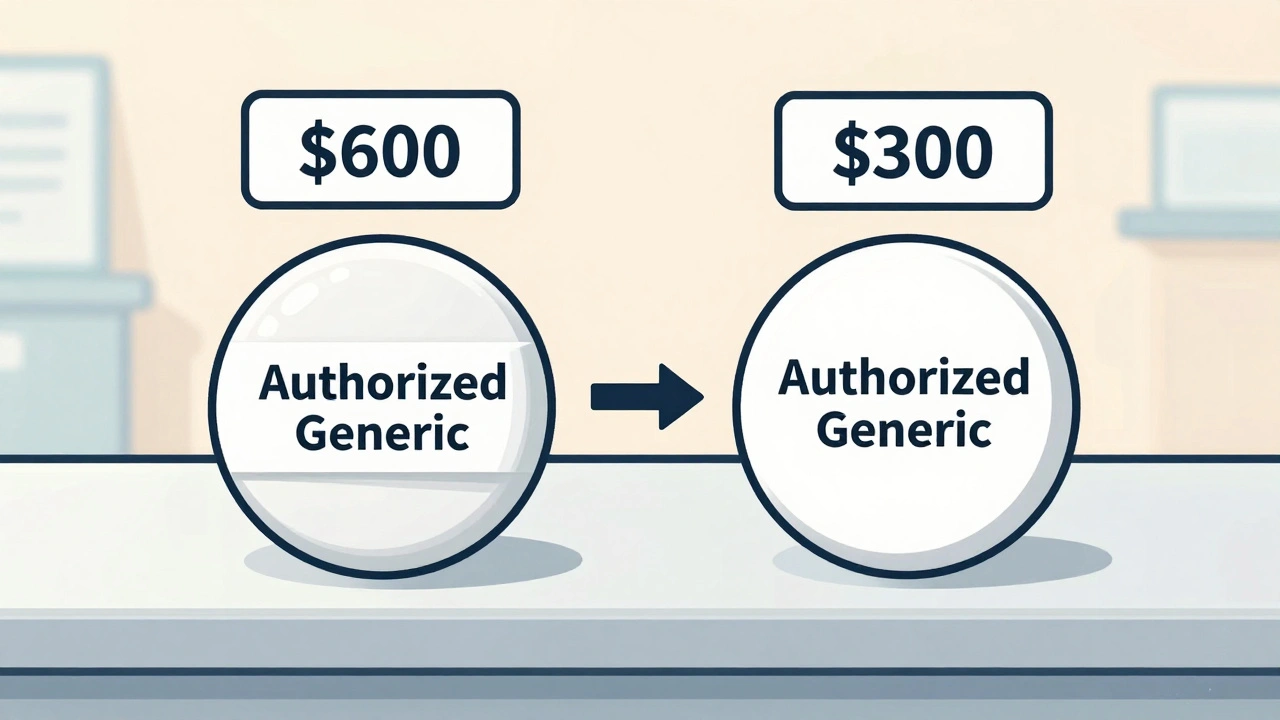
The price drop isn’t because the drug is cheaper to make. It’s because of competition-and the timing of it. Without an authorized generic, the first generic manufacturer would enter the market with no real competition. They’d set a price just below the brand, maybe 20-30% lower. Patients and pharmacies would switch. The brand would lose market share, but slowly. Now, add an authorized generic into the mix. Suddenly, you’ve got two versions of the same drug on the shelf: one from the original brand, one from the brand’s own generic arm. The first generic? They’re now competing with a product that’s identical, but priced lower. To stay in the game, they drop their price too. The result? Retail prices for the brand-name drug fall by 4% to 8%. Wholesale prices for pharmacies drop even more-7% to 14%. Medicaid data shows that when an authorized generic enters the market, the on-invoice price paid by pharmacies drops by 13% to 18%. That’s not a small change. That’s a market reset. Take the EpiPen. In 2016, Mylan raised the price from $100 to $600. Public outrage followed. Then they launched an authorized generic for $300. The brand didn’t drop to $300. But the pressure forced the price down across the board. The same thing happened with Gilead’s hepatitis C drugs Harvoni and Epclusa. Before their patents expired, Gilead released authorized generics to keep market share and prevent wild price swings from competitors.How Authorized Generics Affect Your Pocketbook
Here’s where it gets tricky for patients. Just because an authorized generic is cheaper doesn’t mean you’ll automatically save money. It depends on your pharmacy benefit manager (PBM). PBMs decide which drugs go on which tier in your insurance plan. If your plan puts the brand-name drug and the authorized generic on the same tier, you might pay the same copay for both. That’s confusing. Why would you pay the same for two identical pills? But if the PBM puts the authorized generic on a lower tier-like a preferred generic tier-you’ll pay less. A 2022 analysis of 1.2 million Medicare Part D patients found that when authorized generics were placed on the same tier as regular generics, medication adherence improved by 8.2 percentage points. People filled their prescriptions more often because they could afford them. The problem? Many PBMs still favor the brand-name drug. They get rebates from manufacturers. Those rebates don’t always go to you. They go to the plan. So even if the authorized generic is cheaper, your out-of-pocket cost might not reflect that.
Authorized Generics vs. Regular Generics
Let’s clear up a common mix-up. Regular generics are made by different companies after the brand’s patent expires. They have to prove they’re bioequivalent to the brand. They’re often made in different factories, sometimes overseas. They go through the ANDA process. That takes time. Authorized generics? They’re made by the brand’s own plant. Same batch. Same line. Same quality control. No testing needed. They hit the market on day one of generic competition. That’s why authorized generics are often the first to drop in price. They’re not waiting for regulatory approval. They’re already there. And here’s something most people don’t realize: authorized generics don’t stay expensive. Some worry they’re a way for big pharma to quietly keep prices high. But the Federal Trade Commission studied this for years. Their conclusion? Authorized generics don’t lead to higher prices over time. In fact, they drive prices down faster and harder than regular generics alone.Who Benefits the Most?
Patients with chronic conditions benefit the most. Drugs for high blood pressure, diabetes, depression, cholesterol-these are the ones where authorized generics are most common. If you’re on a long-term medication, even a 10% price drop adds up over a year. Medicare beneficiaries are another big group. With the Inflation Reduction Act capping out-of-pocket drug costs at $2,000 per year for seniors, every dollar saved matters. Authorized generics help keep those costs low. Pharmacies benefit too. When authorized generics are priced lower, they can offer better deals to patients. That builds loyalty. It also helps them avoid being stuck with high Maximum Allowable Cost (MAC) rates set by PBMs, which can leave pharmacies losing money on every prescription they fill.
What You Can Do to Save
You don’t need a PhD to get the best price on your meds. Here’s how:- Ask your pharmacist: “Is there an authorized generic for this drug?”
- Check your insurance formulary. Look for the authorized generic separately from the brand name.
- If your plan doesn’t cover the generic, ask if you can switch. Many plans will approve it if you request it.
- Use price comparison tools like GoodRx or SingleCare. They often list authorized generics at the lowest price.
- Don’t assume the brand is better. If the label says “authorized generic,” it’s the same drug.
Are There Downsides?
There’s one big concern: patent settlements. Sometimes, brand manufacturers pay generic companies to delay their entry into the market. In exchange, they launch an authorized generic to keep the market stable. This is called a “pay-for-delay” deal. The FTC has cracked down on these agreements. They’re illegal if they’re meant to block competition. But they still happen. That’s why authorized generics aren’t always a pure win. Sometimes, they’re part of a bigger game. Still, for most patients, they’re a win. They bring down prices faster. They increase access. And they give you a real alternative to paying full price for a drug you’ve been taking for years.The Bigger Picture
Authorized generics make up about 12% of the $60 billion U.S. generic drug market. That might sound small. But their impact is huge. They force competition before it even starts. They keep prices from spiking. They give patients options when they need them most. And here’s the quiet truth: if you’re paying full price for a brand-name drug, you might be overpaying. Not because the drug is better. But because you don’t know what’s really on the shelf. The next time you get a prescription, ask. Look at the label. Ask your pharmacist. You might be surprised to find out you’ve been paying more than you needed to-for a pill that’s already in your medicine cabinet.Are authorized generics the same as regular generics?
Yes and no. Authorized generics are identical to the brand-name drug in every way-same active ingredient, same manufacturer, same factory. Regular generics are made by different companies after the patent expires and must prove they work the same way. Authorized generics skip the approval process because they’re made under the original brand’s FDA application.
Why don’t all pharmacies carry authorized generics?
It depends on your pharmacy benefit manager (PBM). PBMs decide which drugs are stocked and at what price. Sometimes they favor the brand because of rebates. Other times, they push authorized generics because they’re cheaper. It’s not about the pharmacy-it’s about the plan’s contract with the PBM.
Can I ask my doctor to prescribe an authorized generic?
Yes. Doctors can prescribe by brand or generic name. If your prescription says “brand name only,” you can ask your doctor to change it to “dispense as written” or “generic allowed.” Many will agree, especially if you explain you’re looking to save money.
Do authorized generics have the same side effects as the brand?
Yes. Since they’re made with the same ingredients in the same facility, side effects are identical. The FDA requires this. If you’ve tolerated the brand, you’ll tolerate the authorized generic without issue.
How do I know if my drug has an authorized generic?
Check the FDA’s quarterly list of authorized generics. You can also ask your pharmacist or search online using the drug’s generic name plus “authorized generic.” Many drug information sites, like Drugs.com, list this information too.



Comments
Jennifer Patrician
Okay but let’s be real-this whole ‘authorized generic’ thing is just Big Pharma’s way of keeping you hooked while pretending they’re helping. Same pill, same factory, but now they’re selling it to you at a discount so you don’t notice they’re still raking in cash. It’s not cheaper because of competition-it’s cheaper because they’re manipulating the market. Wake up.
December 5, 2025 AT 01:23
Mellissa Landrum
lol so u mean the same drug in diff bottle is cheaper? yeah right. i bet they just put more filler in it n call it a day. my cousin works at a pharma plant n she says they switch batches n no one checks. #conspiracy
December 6, 2025 AT 15:08
Mark Curry
Interesting. I never thought about it like this. It’s kind of like buying the same car but with a different badge. The engine’s the same, but you pay more for the logo. Makes you wonder how much of what we pay for is just branding. 🤔
December 7, 2025 AT 14:08
Manish Shankar
It is indeed a fascinating development in pharmaceutical economics. The authorized generic model demonstrates a pragmatic alignment of regulatory efficiency with market dynamics. One must acknowledge the structural integrity of the Hatch-Waxman Act in facilitating timely access to affordable therapeutics while preserving quality standards. A commendable innovation, albeit underutilized in public awareness.
December 9, 2025 AT 11:14
luke newton
You people are so naive. This isn’t about savings-it’s about control. They want you to think you’re getting a deal so you don’t question why the brand version still costs $500. They’re not helping you. They’re training you to accept less. And then they raise the price again next year. It’s a trap.
December 10, 2025 AT 02:43
Ali Bradshaw
Love this breakdown. Honestly, I didn’t realize authorized generics were the same pill. I always assumed they were ‘good enough’ but not quite the same. Turns out, I was paying extra for a sticker. Time to ask my pharmacist next time.
December 11, 2025 AT 00:53
an mo
Authorized generics are a regulatory arbitrage mechanism designed to suppress true generic competition by leveraging the NDA’s regulatory moat. PBMs collude with manufacturers to maintain rebate structures that disincentivize substitution, thereby preserving margin integrity over patient welfare. This is not market efficiency-it’s rent-seeking disguised as consumer benefit.
December 11, 2025 AT 02:27
aditya dixit
This is one of those rare cases where capitalism and public health actually align. The authorized generic model reduces price volatility and accelerates affordability without compromising safety. It’s a quiet win for patients, even if the system doesn’t always make it obvious. We should be promoting this model, not doubting it.
December 12, 2025 AT 01:22
Lynette Myles
Same pill. Different label. Still overpriced.
December 12, 2025 AT 19:16
Annie Grajewski
so like… if it’s the same drug, why does the brand still exist? are we just being gaslit by the medical industrial complex? i mean, i get it, i’m not a genius, but even i can tell this smells like a scam. like… why do i need two versions of the same thing? who benefits? not me. definitely not me.
December 13, 2025 AT 02:03
Jimmy Jude
Let me tell you something. I’ve been on the same med for 12 years. I switched to the authorized generic. My insurance didn’t care. My pharmacist didn’t care. My doctor didn’t care. But my body? It didn’t care either. Same results. Same side effects. Same relief. But I paid $12 instead of $120. That’s not a win. That’s a revolution. And nobody’s talking about it. Why? Because they don’t want you to know you’ve been overpaying for a sticker.
December 15, 2025 AT 01:07
Mark Ziegenbein
It’s not just about the pill-it’s about the entire architecture of pharmaceutical capitalism. The authorized generic is a tactical maneuver within a system designed to extract maximum value from human vulnerability. The FDA’s regulatory framework, while ostensibly protective, is structurally complicit in allowing manufacturers to leverage patent law as a shield while simultaneously deploying authorized generics to cannibalize their own market share under the guise of affordability. This isn’t transparency-it’s theater. And we’re all just spectators waiting for the next act where the price ticks back up. The real tragedy? We keep buying the tickets.
December 16, 2025 AT 07:44