Glomerulonephritis: How Your Immune System Attacks Kidney Filters
- Colin Hurd
- 27 December 2025
- 12 Comments
Imagine your kidneys are like a high-precision coffee filter. Tiny holes let waste and water out, but keep protein and blood safely inside. Now imagine your immune system - the very thing meant to protect you - starts attacking that filter. That’s glomerulonephritis. It’s not a single disease. It’s a group of conditions where your body turns on its own kidney filters, causing inflammation, protein leakage, and sometimes, permanent damage.
What Exactly Gets Damaged in Your Kidneys?
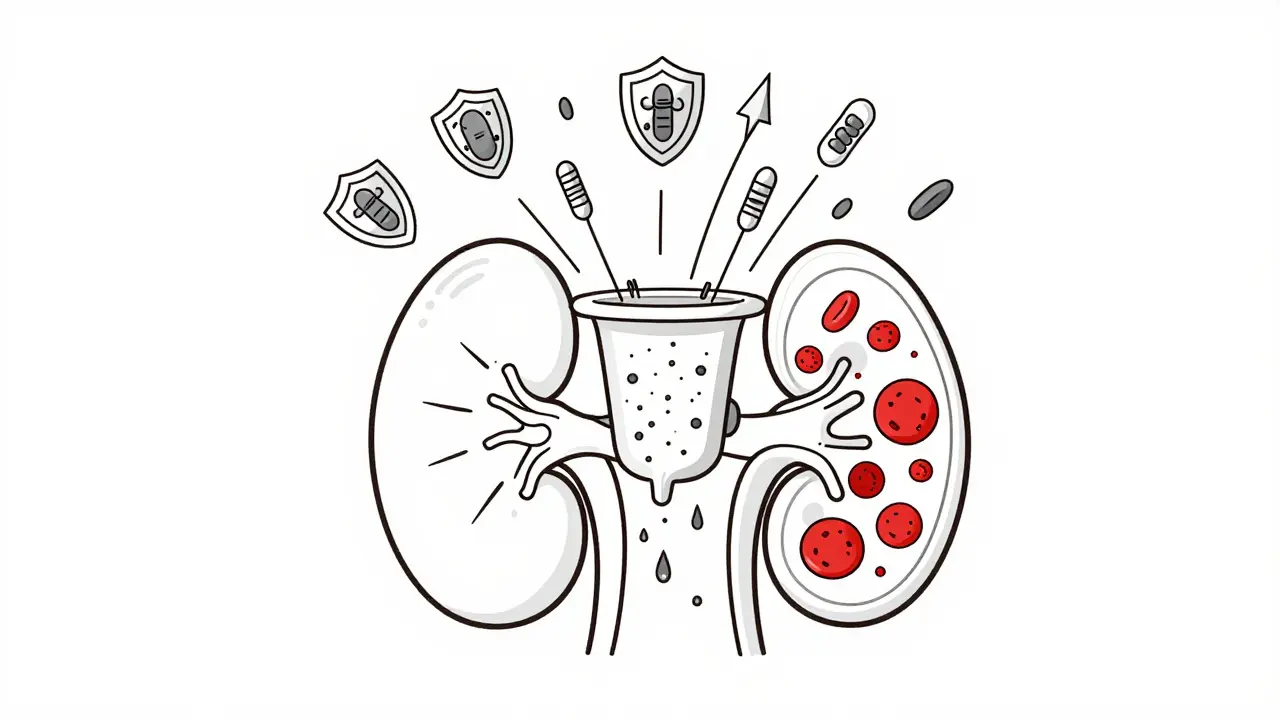
Your kidneys contain about a million tiny filtering units called glomeruli. Each one is made of three layers: the endothelial cells lining the blood vessels, the basement membrane (a structural scaffold), and the podocytes - specialized cells with foot-like projections that wrap around the capillaries. Together, they form a barrier that keeps blood cells and protein in your bloodstream while letting waste flow out as urine. In glomerulonephritis, this barrier breaks down. Immune cells and proteins swarm in, causing swelling and scarring. The result? Blood or protein leaks into your urine. You might not notice it at first. But over time, your kidneys lose their ability to filter properly. That’s when symptoms like swelling in the legs, dark or foamy urine, high blood pressure, and fatigue start showing up.Two Main Ways Your Immune System Goes Wrong
There are two major pathways that trigger this immune attack, and they lead to different types of glomerulonephritis. The first is immune complex-mediated damage. This happens when antibodies bind to foreign invaders - like bacteria or viruses - but then get stuck in the glomeruli. The immune system tries to clear them, but ends up damaging the filter in the process. This is common after strep throat (post-streptococcal GN), or in autoimmune diseases like lupus. About 15% of kids who get strep develop this form, but 95% recover fully with time. The second is complement system dysregulation. This is where your body’s internal alarm system - the complement system - gets stuck on. Normally, it helps destroy pathogens. But in conditions like C3 glomerulonephritis (C3G), it attacks your own glomeruli without any trigger. In C3G, C3 protein builds up in the kidneys at levels three to five times higher than normal. Around 60-70% of these cases are caused by autoantibodies that block the body’s natural brakes on the complement system. This form is rarer - affecting 1-2 in a million people - but often more aggressive.The Most Common Type: IgA Nephropathy
IgA nephropathy is the most common form of glomerulonephritis worldwide. In this condition, an abnormal form of the antibody IgA builds up in the glomeruli. It’s more common in East Asia than in North America, where it affects about 2.5 per 100,000 people each year. Many people don’t even know they have it until they notice blood in their urine after a cold or sore throat. About 20-40% of people with IgA nephropathy will eventually develop kidney failure over 20 years. But here’s the key: early detection and treatment can slow or even stop that progression. Monitoring urine protein levels and blood pressure is critical.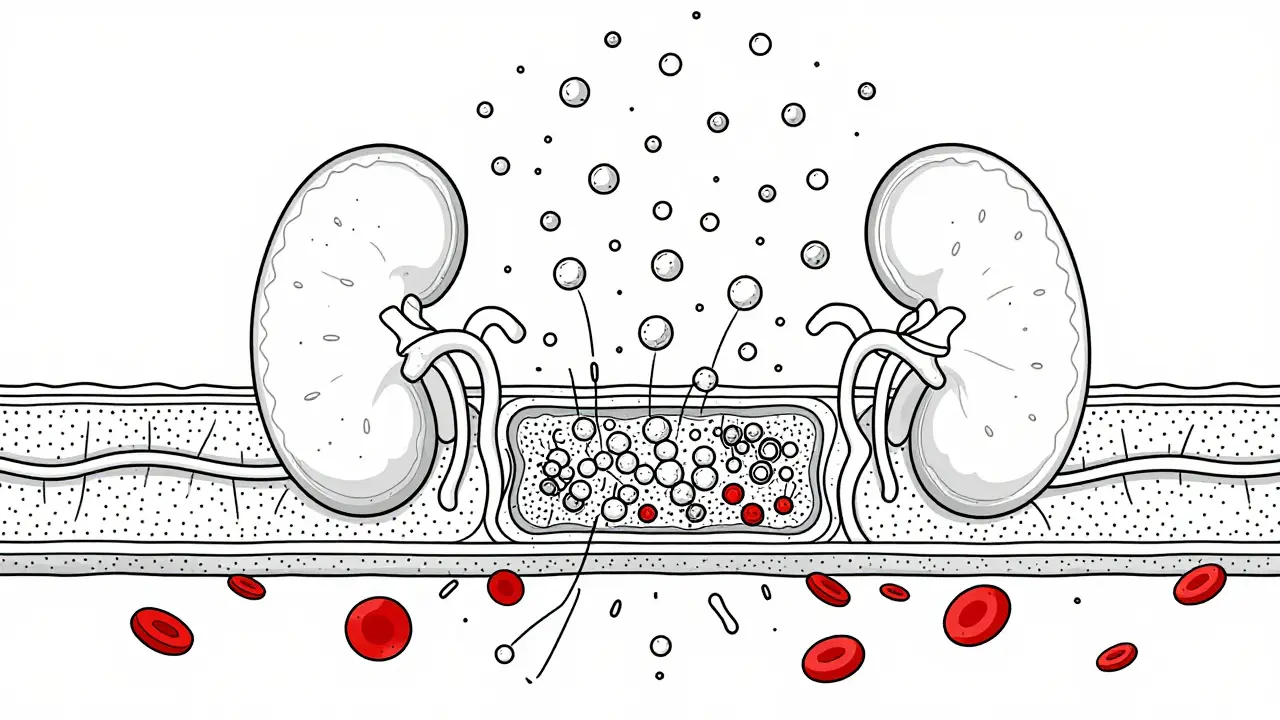
How Is It Diagnosed? It’s Not Simple
You can’t diagnose glomerulonephritis with a simple blood test. That’s why so many people wait months - sometimes over a year - before getting a real answer. The gold standard is a kidney biopsy. A small sample of kidney tissue is taken and examined under a microscope. Pathologists look for patterns: immune deposits, scarring, inflammation. But interpreting these patterns takes years of training. A single biopsy can show different things depending on who reads it. Doctors also check for:- Protein in urine (over 3.5 grams per day means nephrotic syndrome)
- Red blood cells in urine (sign of nephritic syndrome)
- High creatinine levels (1.5-3.0 mg/dL suggests reduced kidney function)
- Low albumin in blood (below 3.0 g/dL)
- High cholesterol (often above 160 mg/dL)
Current Treatments: Steroids and Their Costs
For decades, the go-to treatment has been corticosteroids like prednisone. They work - about 60-80% of patients respond initially. But the side effects are brutal.- 72% gain significant weight
- 35% get serious infections
- 28% lose bone density - some end up with broken vertebrae
New Hope: Targeted Therapies That Don’t Blunt the Whole Immune System
The game is changing. Researchers now understand that different types of glomerulonephritis have different triggers. That means we can start targeting them specifically. For C3G, a drug called iptacopan - approved by the FDA in early 2023 - blocks a key part of the complement system. In trials, it cut proteinuria by 52% in 12 months. That’s huge. But it costs about $500,000 a year. Only a fraction of patients can access it. Another drug, eculizumab, is used for rare forms of GN. It works well - but again, it’s expensive and requires lifelong infusions. What’s exciting is the shift toward personalized medicine. By 2025, doctors will start using genetic tests and protein profiles to match patients with the right drug. One expert predicts this will push success rates from 60-70% to over 85%.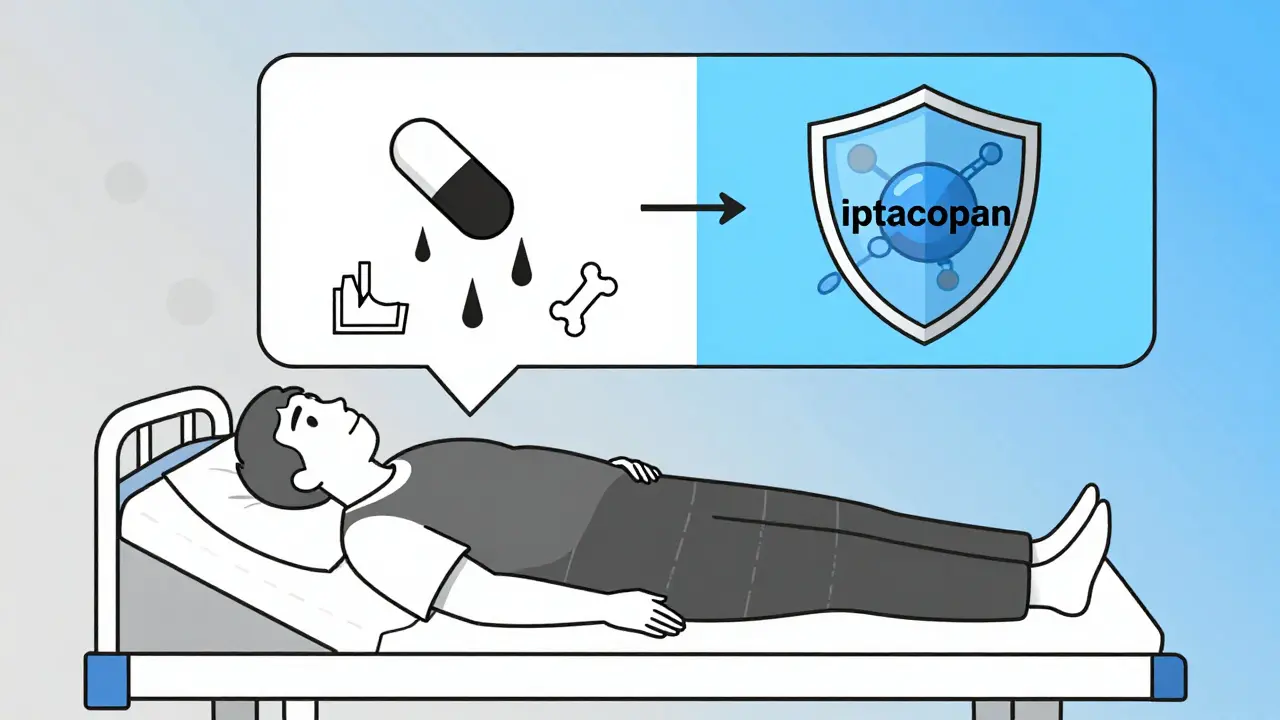
What Patients Are Really Dealing With
Behind the statistics are real people. On forums like Inspire.com and Reddit, patients talk about:- Managing swelling that makes walking hard
- Fear that their next cold could trigger a flare
- Struggling to work because of fatigue
- Worrying if their kids will inherit the disease
What You Can Do Now
If you’ve been told you have protein or blood in your urine, don’t ignore it. See a nephrologist - not just your GP. Early action matters.- Control your blood pressure - aim for under 130/80
- Reduce salt intake - it worsens swelling and raises pressure
- Quit smoking - it speeds up kidney damage
- Get regular urine and blood tests - monitor protein and creatinine
The Bigger Picture
Glomerulonephritis affects 12.5 per 100,000 people in the U.S. each year. It’s responsible for 10-15% of new dialysis cases - about 12,000 to 18,000 people annually. The global market for GN treatments is expected to hit $4.7 billion by 2028. That growth isn’t just about profit. It’s about progress. We’re moving from one-size-fits-all steroids to precision medicine that targets the exact immune flaw in each patient. The future isn’t about shutting down the immune system. It’s about teaching it to stop attacking the kidneys - without leaving you vulnerable to everything else.Is glomerulonephritis curable?
Some forms of glomerulonephritis, like post-streptococcal GN in children, can resolve completely with time. But most chronic forms - like IgA nephropathy or C3G - are not curable. However, they can be managed effectively. Early diagnosis, blood pressure control, and newer targeted therapies can prevent kidney failure in many cases. The goal is to slow or stop progression, not necessarily to reverse damage.
Can glomerulonephritis come back after a transplant?
Yes. In fact, recurrence is common in certain types. IgA nephropathy returns in up to 50% of transplant recipients, sometimes within a few years. C3G has an even higher recurrence rate - about 70-80%. That’s why doctors test for specific immune markers before and after transplant. New drugs like iptacopan are being tested to prevent recurrence, but long-term data is still being gathered.
Does diet affect glomerulonephritis?
Diet won’t cure it, but it can significantly help manage symptoms. A low-sodium diet reduces swelling and helps control blood pressure. Limiting protein intake may ease the kidneys’ workload, especially if kidney function is already reduced. Avoiding processed foods and added sugars helps with cholesterol and inflammation. Working with a renal dietitian is recommended - not a generic "healthy eating" plan.
Are there any natural remedies or supplements that help?
No proven natural remedies cure or significantly improve glomerulonephritis. Some supplements, like fish oil, have been studied for IgA nephropathy with mixed results. But many herbal products can harm the kidneys or interfere with medications. Always talk to your nephrologist before taking anything - even vitamins. What seems harmless could worsen your condition.
How long does it take to get diagnosed?
On average, patients wait 4.2 months from symptom onset to diagnosis. Many see multiple doctors because symptoms like fatigue, swelling, or blood in urine are often mistaken for other issues - like a urinary infection, dehydration, or even stress. A kidney biopsy is required for a definitive diagnosis, and it’s not always ordered right away. If you have persistent symptoms, push for a urine test and referral to a kidney specialist.
Can glomerulonephritis be inherited?
Most forms are not directly inherited. However, some rare types - like Alport syndrome - are genetic. Even in non-genetic forms, family history of autoimmune diseases (like lupus or thyroid disorders) can increase risk. Genetic testing is not routine, but if multiple family members have kidney disease or autoimmune conditions, it’s worth discussing with your doctor.



Comments
Kylie Robson
Glomerulonephritis isn’t just ‘kidney inflammation’-it’s a dysregulated immune cascade targeting the glomerular filtration barrier. The endothelial cells, basement membrane, and podocyte foot processes form a tripartite sieve; when IgA immune complexes or complement C3 convertase go rogue, you get subendothelial or mesangial deposits visible on immunofluorescence. The key is distinguishing between immune-complex-mediated (like post-strep) and complement-mediated (C3G, dense deposit disease) etiologies-biopsy with electron microscopy is non-negotiable. Steroids? They’re blunt instruments. We need C5 inhibitors, factor D blockers, or even anti-CD20 for B-cell-driven forms. Precision nephrology is here, but we’re still stuck in the 1980s with insurance denials.
And yes, IgA nephropathy is the most common GN globally, but its pathogenesis is still poorly understood. The ‘mucosal-immune axis’ hypothesis suggests aberrant glycosylation of IgA1 leads to autoantibody formation. That’s why rituximab trials show promise in refractory cases. But we need better biomarkers than proteinuria alone. Urinary podocyte excretion? NGAL? KIM-1? Those are the future.
Also, don’t let anyone tell you ‘diet cures this.’ Salt restriction helps BP, sure. Protein restriction? Maybe in advanced CKD. But no turmeric, no fish oil, no ‘alkaline diet’ is going to stop complement activation. Don’t fall for wellness influencers.
And iptacopan? $500K/year? That’s a travesty. We need value-based pricing, not patent monopolies.
-Kylie R., Nephrology Fellow
December 29, 2025 AT 08:06
Todd Scott
Man, this post nails it. I’ve been following this since my dad had IgA nephropathy back in the 90s. Back then, they just told him to ‘drink more water’ and gave him steroids that made him look like a moon bear. Fast forward to 2024-now we’ve got drugs that actually target the root cause instead of just turning off the whole immune system like a light switch.
What’s wild is how much the science has shifted. We used to think it was just ‘autoimmune’ and call it a day. Now we’re talking about C3 nephritic factor, factor H autoantibodies, and even complement gene variants. The fact that 70% of C3G patients have a mutation in the complement regulatory pathway? That’s not coincidence-it’s a diagnostic goldmine.
And yeah, the biopsy thing? So true. I’ve seen three different pathologists give three different interpretations on the same slide. One said ‘mild mesangial proliferation,’ another said ‘dense deposit disease,’ third said ‘possible C3GN.’ That’s why we need AI-assisted histopathology. Start training algorithms on biopsy images now, not in 2030.
Also, the fatigue thing? 65% of patients say it’s their worst symptom? That’s the silent killer. Everyone focuses on swelling or proteinuria, but if you’re too tired to get out of bed, you can’t work, you can’t parent, you can’t live. That’s the real cost of this disease.
And the transplant recurrence rates? 50-80%? That’s horrifying. We’re basically transplanting a ticking time bomb. We need prophylactic iptacopan post-transplant. Clinical trials need to start yesterday.
-Todd, former caregiver, current patient advocate
December 30, 2025 AT 17:30
Elizabeth Ganak
So I just found out my brother has IgA nephropathy and honestly I had no idea what that even meant until I read this. Thank you for explaining it so clearly. I’m from India and we don’t talk much about kidney stuff here-it’s always ‘just drink more water’ or ‘take some ayurvedic syrup.’ But now I get it: it’s not about herbs, it’s about the immune system going haywire.
My mom is super worried and wants him to try a ‘detox tea.’ I’m gonna send her this post. Maybe she’ll finally listen. I hope he gets access to the new meds. Here, even regular BP pills are hard to afford.
Hope he’s okay.
-Elizabeth
January 1, 2026 AT 03:06
Robyn Hays
Okay, I’m not a doctor, but I’ve spent 3 years reading every paper, forum, and patient story I could find after my sister was diagnosed with C3G. And honestly? This post feels like someone finally got it right.
It’s not just ‘kidney problems.’ It’s your body’s alarm system screaming in a locked room, and no one’s listening. The complement system? It’s supposed to be your internal SWAT team-zooming in on invaders, zap-zap-zap. But in C3G? It’s like the SWAT team got a glitch and started torching your own house because they thought the smoke alarm was a burglar.
And iptacopan? It’s not a miracle drug-it’s a pause button. But a pause button that lets you breathe again. I’ve seen people go from wheelchair to walking their dog. That’s not science fiction. That’s real.
But the cost? Oh, honey. $500K a year? That’s not medicine. That’s a hostage situation. We’re letting Big Pharma hold patients’ lives ransom while they count their billions.
And the fatigue? Don’t let anyone tell you it’s ‘just in your head.’ I watched my sister sleep 18 hours a day for months. Her brain was just… tired. Not lazy. Tired.
Keep talking. Keep sharing. We’re not rare. We’re just invisible.
-Robyn
January 3, 2026 AT 00:11
Liz MENDOZA
Thank you for writing this. I’ve been silent for two years because I was ashamed. I thought if I just ate better, meditated more, drank celery juice, I could ‘heal’ this. I didn’t want to be ‘that person’ with the chronic illness.
But reading this? It didn’t make me feel broken. It made me feel seen.
I have IgA nephropathy. Diagnosed at 28. Now 31. My protein levels are stable thanks to ACE inhibitors and low salt. I still get fatigued. I still panic when I get a cold. I still cry in the shower sometimes.
But I’m not alone. And I’m not failing. I’m surviving. And that’s enough.
-Liz
January 4, 2026 AT 06:07
Miriam Piro
EVERYTHING YOU SAID IS A LIE. 😈
Glomerulonephritis is caused by 5G radiation + glyphosate + CDC vaccines + fluoride in the water. The ‘biopsy’? That’s just a scam to sell you more drugs. The ‘complement system’? That’s not real science-it’s Big Pharma’s fairy tale to justify $500K pills.
Look up Dr. Robert Young-he proved that alkaline diets cure kidney disease. And the ‘IgA’ nonsense? That’s just your body trying to detox from chemtrails.
My cousin had GN and took turmeric + lemon water + infrared sauna. No steroids. No biopsy. Now she’s hiking in Patagonia. 🌿☀️
Wake up, sheeple. The system doesn’t want you healthy. It wants you dependent. 💉🩸
-Miriam, Truth Seeker 🕵️♀️
January 5, 2026 AT 22:52
Caitlin Foster
WAIT-SO WE’RE TALKING ABOUT A DISEASE WHERE YOUR BODY ATTACKS ITS OWN KIDNEYS LIKE A TERRIBLE EX?!!! 😱
AND THE TREATMENT IS STEROIDS THAT TURN YOU INTO A HUMAN BALLOON?!?!?!
AND THE NEW DRUG COSTS MORE THAN A HOUSE?!?!?!
AND WE STILL DON’T EVEN KNOW WHY IT HAPPENS?!?!?!
WHY ISN’T THIS ON THE FRONT PAGE OF EVERY NEWS SITE?!?!?!
WHY AM I ONLY HEARING ABOUT THIS BECAUSE SOMEONE’S DAD GOT IT?!?!?!
WE NEED A MOVEMENT. A PROTEST. A TIKTOK CHALLENGE. #KidneyBetrayal
-Caitlin, currently Googling ‘how to start a viral health campaign’
January 6, 2026 AT 00:47
Andrew Gurung
Oh, so now we’re pretending this is ‘science’? Please. You’re just rebranding ancient kidney failure with fancy acronyms and billionaire-pharma jargon. C3G? IgA? Who even cares? The real problem? You’re all just terrified of death, so you pay $500K for a drug that buys you 18 more months of fatigue and dialysis.
My uncle had GN. He lived 7 years on dialysis. He never took a single ‘targeted therapy.’ He ate rice, prayed, and died peacefully. No biopsy. No iptacopan. No ‘precision medicine.’ Just dignity.
Stop selling fear. Stop selling hope. Just let people live-or die-with grace.
-Andrew, the only one with real perspective 🙄
January 6, 2026 AT 21:51
Paula Alencar
It is with profound respect for the scientific rigor and clinical insight demonstrated in this exposition that I feel compelled to offer a formal response. The elucidation of glomerular pathophysiology, particularly the distinction between immune-complex-mediated and complement-mediated etiologies, represents a paradigmatic advancement in nephrological understanding.
However, the ethical implications of pharmaceutical pricing mechanisms, particularly the disparity between therapeutic efficacy and socioeconomic accessibility, demand immediate institutional recalibration. The fact that iptacopan, a molecule that demonstrably reduces proteinuria by 52%, remains inaccessible to 90% of global patients due to cost, constitutes not merely a market failure-but a moral failure of the highest order.
Furthermore, the normalization of fatigue as a ‘silent’ symptom underscores a systemic devaluation of patient experience in clinical discourse. To reduce suffering to biomarkers is to dehumanize the very essence of medicine.
Let us not confuse innovation with equity. Let us not mistake precision medicine for justice.
-Paula Alencar, MD, PhD, Medical Ethics Chair, Johns Hopkins
January 7, 2026 AT 23:32
Chris Garcia
As a Nigerian physician who has treated GN patients in Lagos and Abuja, I can tell you: the real tragedy isn’t the lack of drugs-it’s the lack of diagnosis.
Here, we don’t have biopsy machines. We don’t have nephrologists. We don’t have even basic urine dipsticks in 60% of rural clinics. People die because their urine looks ‘normal’ to a general practitioner who thinks it’s ‘just dehydration.’
And yes, iptacopan? It’s science fiction here. We’re fighting for $0.50 test strips, not $500K pills.
But we are building community screening programs. We are training nurses to recognize foamy urine. We are teaching grandmothers to watch for swollen ankles.
Progress isn’t always a new drug. Sometimes, it’s a single person who knows what to look for.
-Chris, from the frontlines
January 8, 2026 AT 16:41
James Bowers
There is no such thing as ‘cure.’ There is only management. There is no ‘precision medicine’-only expensive palliation. The entire narrative of glomerulonephritis is constructed to sustain a medical-industrial complex that profits from chronicity.
Biopsy? Steroids? Iptacopan? All are distractions from the root: systemic inflammation driven by diet, pollution, and sedentary lifestyle.
Stop chasing molecules. Start chasing lifestyle. The data is clear: populations with low processed food intake have lower GN incidence.
-James Bowers, MD, Board Certified, Retired
January 10, 2026 AT 04:49
Will Neitzer
While the preceding comments reflect a broad spectrum of perspectives-from the clinically rigorous to the emotionally resonant-I feel compelled to underscore the necessity of a unified, evidence-based framework for patient advocacy. The data presented in the original post is methodologically sound and aligns with current guidelines from KDIGO and the American Society of Nephrology.
That said, the disparity in access to targeted therapies is not merely a logistical challenge; it is a failure of distributive justice in global health policy. The fact that C3G, a condition with a known genetic and immunological signature, remains untreatable for the majority of affected individuals is not a scientific limitation-it is a political one.
Moreover, the normalization of fatigue as a ‘minor’ symptom in clinical discourse must be rectified. Patient-reported outcomes, when validated and integrated into trial design, are not ancillary-they are primary.
Let us not mistake advocacy for activism, nor compassion for sentimentality. The path forward requires policy, precision, and persistence.
-Will Neitzer, MD, PhD, Clinical Research Director, Mayo Clinic
January 10, 2026 AT 04:55