How Long Does the FDA Take to Approve Generic Drugs? 2025 Timelines Explained
- Colin Hurd
- 18 November 2025
- 12 Comments
When you pick up a generic pill at the pharmacy, you might not think about how long it took to get there. But behind every affordable generic drug is a complex, tightly timed review process by the U.S. Food and Drug Administration (FDA). For manufacturers, patients, and pharmacies, understanding how long this takes isn’t just paperwork-it affects access, cost, and even lives.
What’s the standard timeline for FDA generic drug approval?
The FDA targets a 10-month review period for standard generic drug applications, starting from the day they accept your Abbreviated New Drug Application (ANDA). This isn’t a guarantee-it’s a goal. And in 2025, they’re getting closer to hitting it.
According to the latest FDA quarterly reports, the average time to approve a generic drug has dropped to 35.6 days for the mean approval time and 25.3 days for the median. That’s a big shift from just five years ago, when approvals averaged over 40 days. The reason? The Generic Drug User Fee Amendments (GDUFA), which gave the FDA funding and clear performance targets to speed things up.
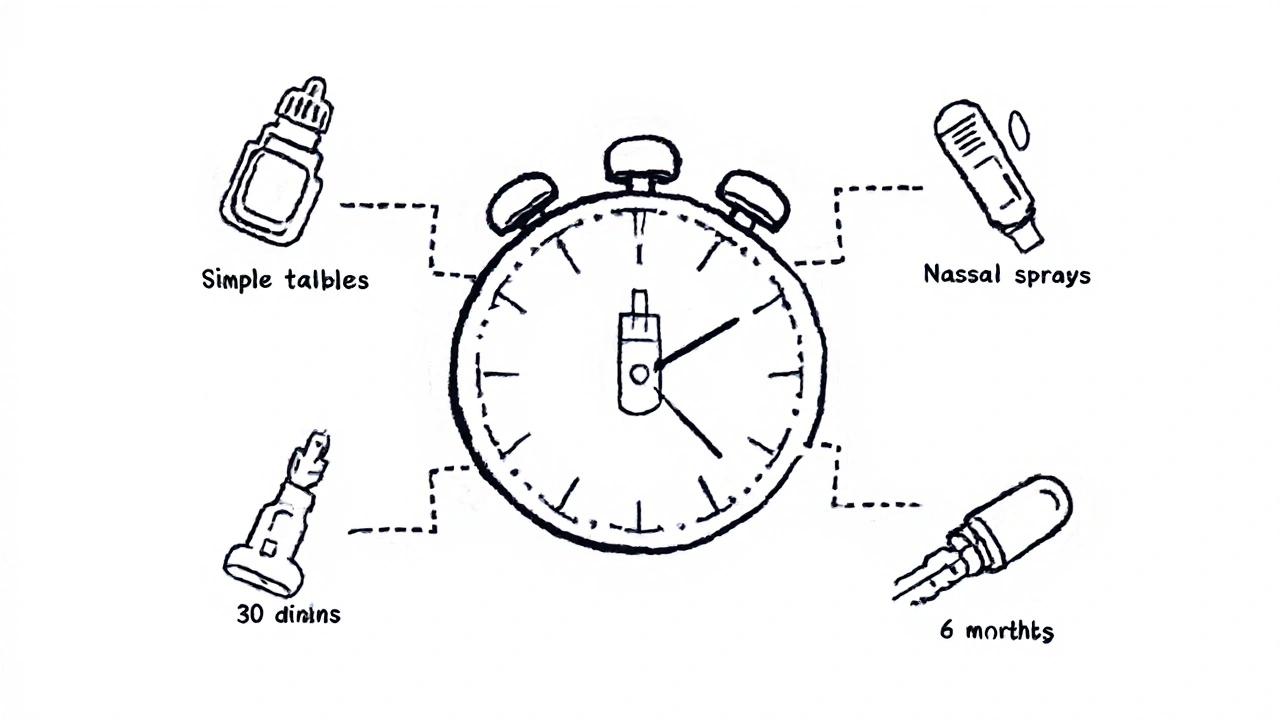
But here’s the catch: those numbers are averages. They don’t tell the full story. A simple tablet form of a common drug like ibuprofen might clear approval in under 30 days. A complex nasal spray, injectable, or extended-release capsule? That could take 8 to 12 months-or longer.
Why do some generic drugs take so much longer?
Not all generics are created equal. The FDA breaks them into two categories: simple and complex.
Simple generics are tablets or capsules with well-understood chemistry. Think metformin, lisinopril, or amoxicillin. These are the low-hanging fruit. They’ve been around for decades. The FDA has decades of data on them. Manufacturing processes are standardized. These make up the bulk of approvals-and they’re the ones moving fastest.
Complex generics are trickier. These include inhalers, topical creams, injectables, and drugs with special delivery systems. For example, approving a generic version of an insulin pen or a chemotherapy infusion requires proving it behaves *exactly* like the brand-name drug in the body-not just in a test tube, but in real human physiology. That’s harder to prove. It needs more testing, more data, and more scrutiny.
The FDA’s Complex Generic Drug Products initiative, launched in 2023, assigned dedicated review teams to these tougher applications. Since then, approval times for complex generics have dropped by 22%. Still, they’re not catching up to simple ones. One Reddit user shared their experience: a standard tablet took 278 days. A nasal spray? 1,087 days. That’s nearly three years.
What’s a “first generic” and why does it matter?
The FDA gives special attention to “first generics”-the very first version of a brand-name drug to hit the market after its patent expires. These are critical. They trigger price drops across the board. When the first generic enters, prices often drop by 80% within a year.
In 2025, the FDA approved 11 first generics in the first nine months-pacing ahead of 2024. Some of the most notable: Epinephrine Injection, Bosentan Tablet, Ivermectin Tablet, and Doxycycline Hyclate Oral Suspension. These aren’t just drugs-they’re lifelines for people who couldn’t afford the brand versions.
The FDA prioritizes these applications. They’re flagged for faster review, and manufacturers can request a pre-submission meeting to get feedback before even filing. That’s a game-changer. It reduces the chance of a complete response letter (CRL)-a formal notice that the application is missing something.

What happens if the FDA sends a complete response letter?
Even with the best application, 42.3% of ANDAs get a complete response letter in the first round. That’s up from 37.8% in 2024. It doesn’t mean rejection. It means: “We need more info.”
Common reasons? Incomplete chemistry data, unclear manufacturing controls, missing bioequivalence studies, or unresolved issues from pre-approval inspections. If you get a CRL, you have to fix it and resubmit. That adds 3 to 6 months to the timeline-sometimes more.
Manufacturers who invest in high-quality submissions upfront-clean data, precise specifications, and full transparency-see far fewer CRLs. The American Association of Pharmaceutical Scientists recommends submitting “complete chemistry, manufacturing, and controls” (CMC) data from day one. It’s expensive. It’s time-consuming. But it saves time in the long run.
How does priority review work for generics?
Unlike new drugs, the FDA doesn’t have a formal “priority review” category for generics. But they do have workarounds.
If a generic drug treats a shortage, or if there’s no other generic on the market, the FDA can fast-track it. In 2025, the agency approved 27 generics under “drug shortage” priority. These often clear review in under 6 months.
There’s also a new pilot program called the Commissioner’s National Priority Voucher (CNPV). If approved, it could slash review times from 10 months to just 1-2 months for select applications. It’s still in testing, but early results show promise. The FDA is also using AI to scan applications faster. In pilot programs, AI reduced review time by 15.8% for standard generics.
How do fees and funding affect approval speed?
The FDA doesn’t use taxpayer money alone to review generics. Under GDUFA, manufacturers pay user fees-$138,400 per ANDA in 2025. That money funds reviewers, inspectors, and technology. Without it, the timeline would crawl back to where it was in 2010: 20+ months.
Small businesses and first-time applicants can apply for fee waivers. But only 4.7% of applications qualify. Most are from big players like Teva, Viatris, and Sandoz-companies that have teams dedicated to navigating the FDA’s system. They know the rules. They know who to talk to. They submit early and often.
That creates a gap. Big companies get faster approvals. Smaller ones struggle. The FDA says they’re trying to level the playing field with guidance documents and pre-submission meetings. But in practice, experience still matters.
What’s the real-world impact of faster approvals?
Speed isn’t just about paperwork. It’s about money-and health.
Generic drugs make up 90% of all prescriptions in the U.S. But they cost only 23% of what brand-name drugs do. Over the past decade, FDA-approved generics have saved the healthcare system $1.7 trillion. That’s more than the GDP of most countries.
Every time a generic hits the market, insurers pay less. Patients pay less. Hospitals save on budgets. And when a drug is in shortage-like insulin or antibiotics-faster approval means people don’t go without.
IQVIA projects the generic market will grow 6.2% per year through 2030. Why? Because the FDA is getting better at approving them. And patients are demanding cheaper options.
What’s next for FDA generic approvals?
The FDA’s GDUFA III goals (2023-2027) aim for 90% of standard applications approved on time, and 75% of priority ones. By 2027, they’re targeting a median approval time of just 20 days for standard generics and 10 days for priority ones.
That’s ambitious. And controversial. Some experts warn that rushing approvals could risk quality. Dr. Peter Lurie from the Center for Science in the Public Interest says: “Rushing without adequate oversight puts patients at risk.”
The FDA responds with a risk-based approach: more scrutiny on high-risk products, less on low-risk ones. They’re also using real-time data from manufacturing sites and AI to spot red flags faster.
For now, the trend is clear: approvals are getting faster. And for the millions who rely on generics, that’s not just policy-it’s medicine.



Comments
Dana Dolan
I just got my generic metformin last week and it cost me $4. Like, how is this even legal? Brand name was $120. The FDA’s doing something right.
November 19, 2025 AT 16:25
Codie Wagers
Let’s be real-this whole system is a charade. They call it 'faster approval' but they’re just greenlighting half-baked generics so Big Pharma can keep their monopoly pricing on the brand. You think a 35-day approval means quality? It means they stopped caring.
November 19, 2025 AT 21:55
Paige Lund
So let me get this straight… you’re telling me the same pill I’ve been taking for 5 years now is suddenly approved in 27 days? I’m not mad, I’m just confused. Did they just skip the whole testing thing?
November 21, 2025 AT 14:07
Michael Salmon
You call that fast? I’ve seen generics take 2 years just because some intern mislabeled a batch number. The whole system is a circus run by people who think ‘CMC’ stands for ‘Can’t Make Sense’.
November 22, 2025 AT 02:05
Joe Durham
I appreciate the transparency here. It’s easy to get frustrated with the system, but the fact that they’re using AI and dedicating teams to complex drugs shows they’re trying. Not perfect, but trying.
November 23, 2025 AT 12:24
Derron Vanderpoel
I had a cousin who couldn’t afford her insulin until the generic came out. Took 14 months. She cried when she saw the price tag. I’m glad the FDA’s speeding up, but… 14 months? That’s too long for someone who’s just trying to live.
November 25, 2025 AT 00:43
river weiss
The GDUFA funding model is essential. Without industry fees, the FDA would be understaffed and overwhelmed. However, fee waivers for small manufacturers must be expanded. Innovation shouldn't be reserved for corporations with legal departments. The current system favors scale over substance.
November 26, 2025 AT 11:22
Brian Rono
They’re calling this progress? Ha. They’re just playing whack-a-mole with the paperwork. Meanwhile, people are dying because the FDA took 18 months to approve a generic epinephrine auto-injector. That’s not efficiency-that’s negligence dressed up in jargon.
November 28, 2025 AT 10:32
seamus moginie
I reckon the FDA's got the right idea but they're still stuck in the 90s. If they used blockchain for batch tracking and AI to flag dodgy data, we'd be talking weeks, not months. Just saying.
November 29, 2025 AT 03:37
Chuck Coffer
Funny how the ‘fast track’ only applies when it’s a big company with a team of lawyers. Small labs? They get buried under CRLs. This isn’t about speed-it’s about who you know.
November 30, 2025 AT 01:53
Marjorie Antoniou
I’ve worked in pharmacy for 15 years. I’ve seen the shift. The cheaper generics aren’t just saving money-they’re saving lives. People who skipped doses because of cost? Now they’re taking them. That’s the real win.
December 1, 2025 AT 00:45
Andrew Baggley
I’m not saying the FDA is perfect, but if they can cut approval time in half without increasing recalls, then they’re doing better than most government agencies. Let’s not throw the baby out with the bathwater.
December 1, 2025 AT 19:42