Drug Patents Explained: How They Control Prices, Generics, and Access
When you hear drug patents, legal protections that let pharmaceutical companies be the only ones to sell a new medicine for a set time. Also known as pharmaceutical patents, they're the reason some pills cost hundreds of dollars—and why others later cost just a few cents. These patents aren’t just paperwork; they’re the engine behind how drugs reach the market, how much you pay for them, and when cheaper versions become available.
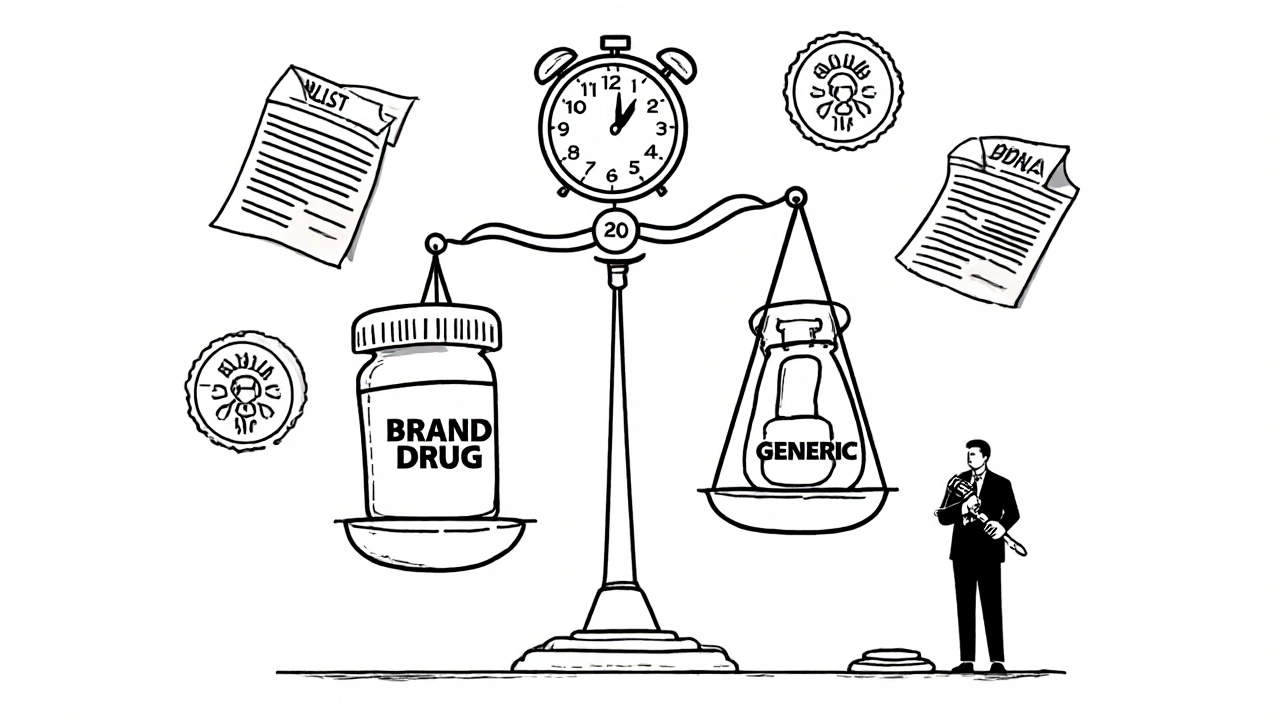
Every new drug starts with a patent that typically lasts 20 years from the date it’s filed. But here’s the catch: by the time a drug gets FDA approval, half that time is often already gone. That’s why companies stretch patents with small changes—new dosages, delivery methods, or combo pills—called evergreening, strategies to extend market exclusivity without adding real clinical benefit. Meanwhile, generic drugs, chemically identical versions of brand-name drugs that enter the market after patent expiration can drop prices by 80% or more. You see this in posts about insulin, cephalexin, and ivermectin—where generic versions are the only affordable option for millions.
Drug patents also shape what treatments are even available. If a patent blocks generics, patients and insurers pay more. But when a patent expires, like with drug patents on popular medications, it triggers a flood of cheaper alternatives. That’s why the FDA’s approval timeline for generics matters so much. And it’s why blockchain and drug verification tools are rising—to make sure those cheap generics aren’t fake. You’ll find posts here that dig into how long FDA approval takes, why generics cost less, and how companies fight to keep prices high even after patents should’ve ended.
Understanding drug patents helps you make smarter choices. It explains why your prescription suddenly got cheaper, why some drugs have no alternatives, and why new treatments take so long to become affordable. What follows is a collection of real-world examples—how patents affect diabetes meds, antibiotics, HIV drugs, and even mental health treatments. You’ll see how patent timing connects to cost, access, and safety. No theory. No fluff. Just what actually happens when a patent runs out—and who wins or loses when it does.
- Colin Hurd
- Feb, 27 2026
- 3 Comments
Future Economic Trends: Forecasts for Generic Drug Markets
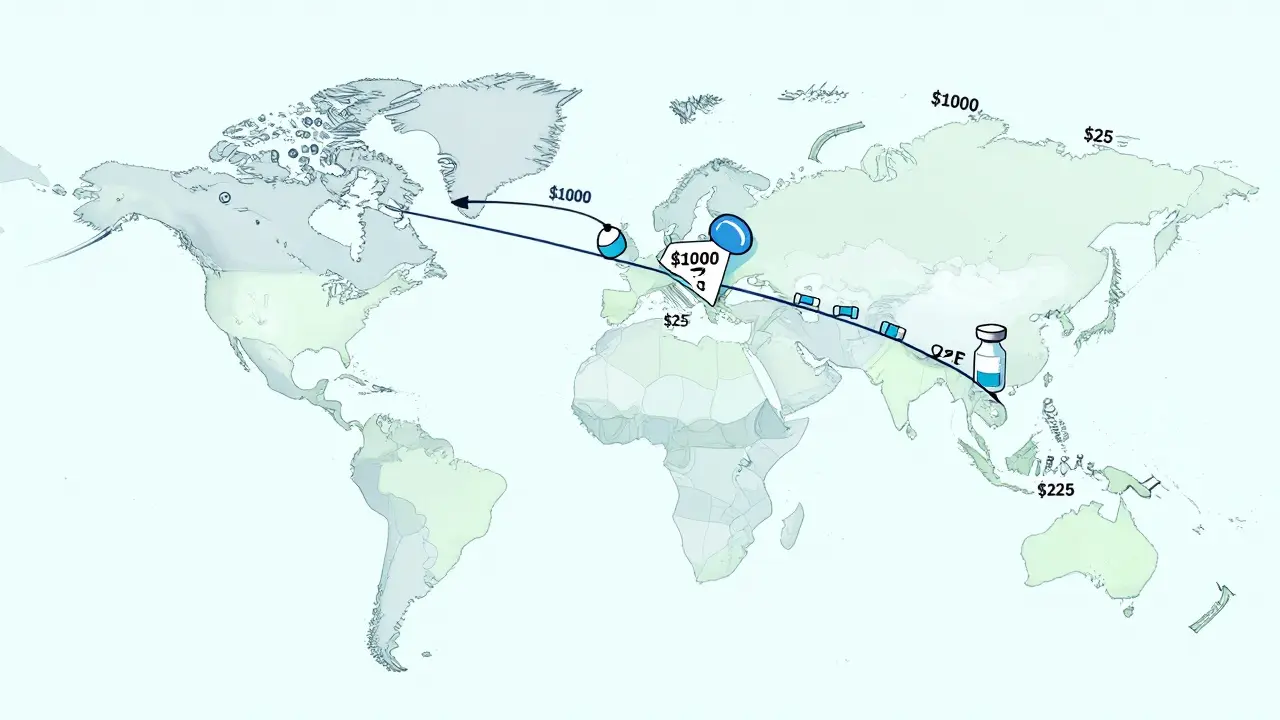
The global generic drug market is set to grow to over $700 billion by 2030, driven by patent expirations, aging populations, and cost pressures. Biosimilars, India, and China are leading the charge, making life-saving medications more affordable worldwide.
- Colin Hurd
- Nov, 20 2025
- 8 Comments
Patent Law and Generics: How Patents Protect Innovation in Pharmaceutical Drugs
Patent law protects pharmaceutical innovation by granting temporary monopolies, but also enables affordable generics through the Hatch-Waxman Act. This balance drives medical progress while saving billions in healthcare costs.