Generic Drug Availability: Why the Same Medicine Costs Different Amounts Around the World
- Colin Hurd
- 24 December 2025
- 10 Comments
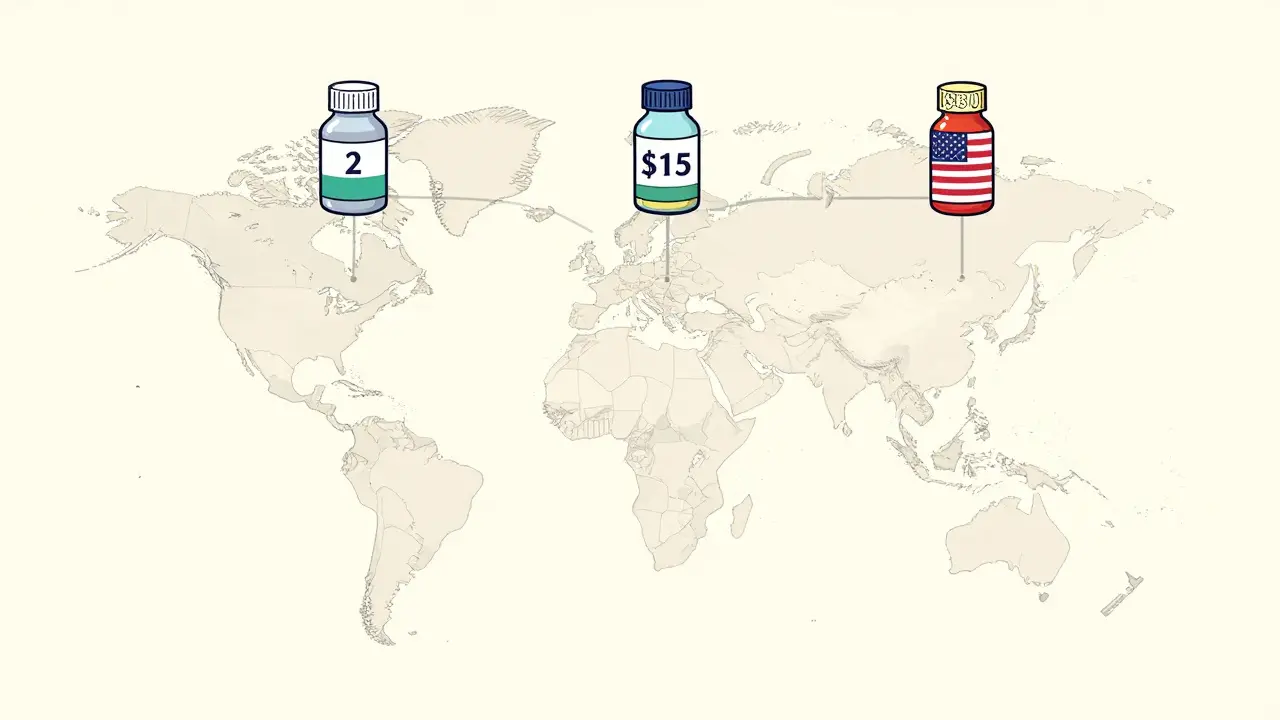
Have you ever traveled abroad and found your prescription medication isn’t available-or costs five times more-than it does at home? It’s not a glitch in your insurance. It’s the reality of how generic drugs are handled differently across the world. The same pill, made by the same factory, can be sold for $2 in India, $15 in Germany, and $80 in the United States. And in some countries, doctors won’t even prescribe it at all.
Why Some Countries Use Generics Like Toothpaste-And Others Treat Them Like Luxury Goods
In the United Kingdom, nearly 83 out of every 100 prescriptions are filled with generic drugs. In Switzerland, that number is just 17. Why such a huge gap? It’s not about how sick people are. It’s about policy, culture, and money. The UK pushes generics hard. Pharmacists are legally allowed to swap brand-name drugs for cheaper generics unless the doctor says no. Patients are educated about safety. Reimbursement systems reward pharmacies for choosing lower-cost options. The result? Billions saved every year. Switzerland? Doctors there still prefer brand-name drugs. Patients trust them more. Insurance pays the same whether you get the brand or the generic. So why switch? Many don’t. And because there’s little pressure to change, generic manufacturers don’t rush in with lower prices. The same drug can cost over six times more in Switzerland than in the UK. It’s not just Europe. In South Korea, even when generic versions of a drug are 50% cheaper, doctors and patients still choose the brand. In the U.S., 90% of prescriptions are generics-but prices are still among the highest in the world. How? Because competition doesn’t always mean lower prices. Sometimes, it means fewer companies left standing-and those that remain raise prices.The U.S. Paradox: High Generic Use, High Prices
The U.S. leads the world in generic prescriptions. Over 90% of all prescriptions are for generics. But the U.S. also pays the most for them. A 30-day supply of metformin, a common diabetes drug, costs about $10 in Canada and $4 in India. In the U.S., it’s $15-$30, sometimes more. Why? Because the market isn’t truly competitive. When a patent expires, dozens of companies should jump in and drive prices down. But in practice, only a few do. And when one manufacturer shuts down production-due to low margins or quality issues-the price spikes. In 2023, the FDA recorded 147 generic drug shortages. Nearly 70% of those came from just one or two factories, mostly in India and China. Even when multiple companies make the same drug, prices don’t always drop. A 2022 MIT study found that some generic drugs-like older antibiotics or blood pressure pills-had sudden, unexplained price hikes, even with six or seven manufacturers selling them. That’s not competition. That’s market failure.India: The Pharmacy of the World-and the Quality Question
India produces about 20% of all generic drugs worldwide. It supplies 40% of the generic medicines used in the U.S. There are over 750 Indian factories approved by the FDA. That’s more than any other country. But here’s the catch: not all Indian-made generics are equal. A 2023 study from Ohio State University found that generic drugs made in India were linked to 54% more severe side effects-including hospitalizations and deaths-than identical drugs made in the U.S. The difference? Quality control. Some factories cut corners to keep prices low. And when you’re making millions of pills for pennies each, cutting corners is tempting. Patients in the U.S. have reported strange reactions after switching to an Indian-made version of levothyroxine or metformin. Not because the active ingredient changed. But because the fillers, coatings, or manufacturing process did. These aren’t fake drugs. They’re legal. But they’re not always identical in how the body reacts to them.
Europe’s Patchwork: 27 Countries, 27 Rules
The European Union has one drug approval agency-the EMA-but each country sets its own pricing, reimbursement, and substitution rules. That means a drug approved in Brussels might take two years to appear on pharmacy shelves in Italy. Germany and the Netherlands have mandatory generic substitution. Pharmacists switch automatically. Patients get lower co-pays. Generic use is over 70%. Italy and Greece? No mandatory substitution. Doctors prescribe brands. Patients pay more. Generic use is below 25%. The result? Italy spends nearly twice as much per person on medicines as Germany, even though they use the same drugs. And then there’s parallel trade. A drug bought cheaply in Poland might be shipped to France and sold at a profit. It’s legal. It saves money. But it also creates supply chain chaos. Pharmacies run out of stock. Patients get confused. And regulators struggle to track where each batch came from.What Happens When a Country Blocks Generic Imports?
In 2020, India banned exports of 26 active pharmaceutical ingredients during the pandemic. Those were raw materials used to make antibiotics, blood pressure pills, and antifungals. Within weeks, hospitals in the U.S., Canada, Australia, and across Europe started running low. That wasn’t an accident. It was a strategic move. India controls the supply. When it shuts off the tap, the world feels it. The U.S. FDA responded by speeding up inspections and approving new suppliers. But it took months. And in the meantime, patients went without. Some switched to more expensive brand-name drugs. Others skipped doses. One study estimated that 12% of patients with chronic conditions skipped or cut their meds during those shortages.Why Don’t We Just Make One Global Standard?
You’d think the world would agree on one set of rules for generic drugs. Same testing. Same quality. Same prices. But that’s not how it works. The FDA requires generics to match brand-name drugs within 80-125% of the same pharmacokinetic levels. The EMA uses a similar range-but not exactly the same tests. India follows WHO guidelines. China has its own standards. And no one wants to give up control. Regulators fear that harmonizing standards might lower quality. Manufacturers fear it might force them to spend more. Governments fear losing power over pricing. The result? A mess. A patient in Australia might get a generic made in India. One in Canada gets the same pill from a factory in Germany. One in the U.S. gets it from a plant in Puerto Rico. All labeled the same. But not all made the same.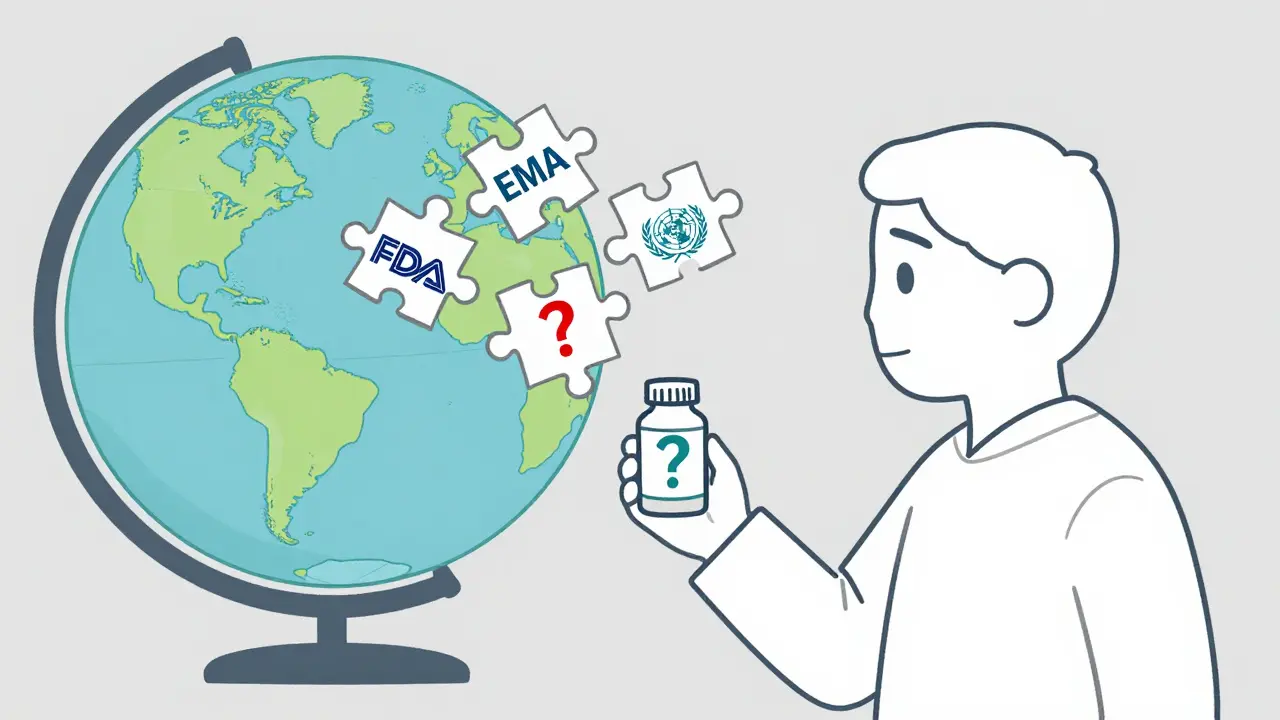
What You Can Do If You Travel or Buy Online
If you’re traveling and need your medication:- Bring enough for your whole trip-just in case.
- Know the generic name of your drug (e.g., “metformin,” not “Glucophage”).
- Ask a local pharmacist if the generic is the same. Don’t assume.
- Avoid buying from unverified online pharmacies. Some sell fake or low-quality generics.
- Check if the pharmacy is verified by PharmacyChecker or LegitScript.
- Look for the manufacturer name. If it’s Cipla, Sun Pharma, or Dr. Reddy’s-those are major Indian companies with FDA approval.
- Watch for side effects after switching. If you feel different, talk to your doctor.


Comments
Winni Victor
So let me get this straight - we’re paying $30 for metformin because some CEO in New Jersey thinks it’s ‘strategic pricing’? Meanwhile, my cousin in Delhi buys the exact same pill for $1.50 and doesn’t even blink. This isn’t capitalism. This is a hostage situation where the hostages are diabetics and the kidnappers are shareholders.
And don’t even get me started on how the FDA approves these Indian generics like they’re ordering takeout. ‘Oh yes, that factory has a 78% compliance rate? Perfect. Ship it.’
I swear, if I had a dollar for every time I heard ‘it’s the same active ingredient,’ I could buy my entire prescription out of pocket. But nope. I’m still stuck paying for the privilege of hoping my body doesn’t revolt.
And yes, I’ve switched brands three times. Each time I felt like a lab rat. One made me dizzy. One gave me acid reflux. One just made me cry in the shower. All labeled ‘metformin.’
Who’s the real villain here? The Indian factory? Or the U.S. system that lets them get away with it? I’m not sure anymore. But I know I’m tired of being the collateral damage.
Also - why do we still act like ‘generic’ means ‘safe’? It means ‘cheaper.’ Big difference. And if you’re not paying attention, your body finds out the hard way.
December 25, 2025 AT 00:24
Linda B.
The FDA is a puppet of Big Pharma and the Indian manufacturing cartel
They let in drugs with unapproved fillers because they’re paid off
Watch what happens when the next pandemic hits and the supply chain ‘breaks’
It’s all planned
They want you dependent on their pills
And they want you too sick to notice
December 25, 2025 AT 18:32
Christopher King
Let’s be real - this whole system is a psychological experiment in mass compliance.
We’ve been trained to believe ‘generic = cheap = bad’ even when the science says otherwise. But then we’re shocked when we pay $80 for a pill that’s identical to one sold for $3 in Thailand?
It’s not about medicine. It’s about control. The system wants you to feel powerless. It wants you to accept that your health is a commodity.
And the worst part? We’ve been sold the lie that ‘competition lowers prices.’ But when only three companies control 90% of the market? That’s not competition. That’s collusion with a side of bureaucracy.
Remember when insulin was $300? Now it’s $25. Why? Because people screamed. Not because the system changed.
Same thing here. The only thing that changes prices is public rage.
So ask yourself - are you angry enough to fight? Or are you just gonna keep swallowing the bill?
And if you think this is about ‘quality’ - then why does the same factory make pills for Europe, the U.S., and India? Why do only the U.S. pills cost 10x more?
It’s not the pill. It’s the price tag. And that’s politics. Not science.
December 25, 2025 AT 23:08
Katherine Blumhardt
okay so i just got back from my trip to mexico and i bought my blood pressure med there for 12 bucks and now i feel kinda weird like my head is floating and i dont know if its the med or just jet lag but also my pharmacist in texas said the indian ones are fine but then my aunt had a heart thing so idk
also can we talk about how no one ever tells you that generics can have different fillers like i thought they were all the same but turns out its like buying a burger with the same patty but different buns and one gives you gas and the other makes you cry
also why is there no app for this like a generic drug scanner or something
also why does my insurance only cover one brand and it costs 40 bucks and i just want to die
December 26, 2025 AT 07:02
sagar patel
India makes 20% of the world’s generics. We don’t cut corners. We optimize. The U.S. pays for brand loyalty. We pay for survival.
Yes, some factories have issues. But so do yours. The FDA caught 147 shortages - how many were from U.S. plants? Zero. Because they don’t exist anymore.
Our workers don’t get bonuses. They get meals. We don’t have shareholders screaming for quarterly profits. We have families feeding on this industry.
When your country blames us for price hikes, remember - we’re the ones making the pills while your CEOs buy private jets.
And if you want quality? Test it. Don’t just scream ‘Indian = dangerous.’
We’re not the problem. You’re just not paying attention.
December 26, 2025 AT 21:15
Harbans Singh
I’ve worked in pharma supply chains in both India and the U.S. and I can tell you - the gap isn’t about evil corporations or lazy regulators.
It’s about infrastructure. In India, we produce at scale because we have to. No one has insurance. No one can pay $50 for a pill. So we make it cheap, efficient, and damn good.
In the U.S., the system rewards complexity. Brand names get marketing budgets. Pharmacists get paid to push them. Doctors get samples. Generics? They’re an afterthought.
But here’s the thing - the science is there. Most generics work fine. The side effects? Often psychological. Or from switching too fast.
What we need isn’t blame. It’s transparency. Batch numbers. Public databases. A simple QR code on the bottle that tells you where it came from, when it was made, and who tested it.
That’s not hard. It’s just not profitable.
And honestly? If you’re worried about quality, ask your pharmacist for the manufacturer name. Cipla, Sun Pharma, Hetero - they’re the good ones. Not all Indian companies are equal. Same as in the U.S.
We’re all just trying to stay alive. Let’s stop treating each other like enemies.
December 27, 2025 AT 14:13
Oluwatosin Ayodele
You think this is bad? Wait until Africa starts importing generics from China. No FDA. No EMA. Just shipping containers with labels in Mandarin.
Right now, Nigeria gets 60% of its medicines from unregulated sources. People die. Not because of the drug. Because of the packaging. Or the expiration date. Or the fact that the pill is shaped like a heart but contains chalk.
And you Americans are complaining about $15 metformin?
You have the infrastructure. You have the regulators. You have the money.
Stop acting like victims. Start demanding accountability.
And if you want to know why India doesn’t fix every bad factory? Because you’re the ones buying from them. You’re the customers. You’re the ones enabling it.
December 29, 2025 AT 06:23
Jason Jasper
I’ve been on the same generic for 8 years. Never had an issue. Then last year I switched to a new batch - felt off for two weeks. Got my doctor to switch me back. Fine.
My point? It’s not black and white.
Some generics are perfect. Some cause weird reactions. Sometimes it’s the filler. Sometimes it’s your body changing. Sometimes it’s just bad luck.
But the system? It’s broken. Not because of India. Not because of the FDA. Because no one’s tracking the supply chain end-to-end.
Imagine if every pill had a blockchain ID. You scan it, you see where it came from, who tested it, when it shipped.
That’s not sci-fi. It’s possible.
But nobody wants to pay for it.
So we get chaos.
And we blame the wrong people.
December 29, 2025 AT 12:42
Mussin Machhour
Bro. Just buy your meds from Canada. Seriously. The same pills. Half the price. No drama.
I’ve been doing it for years. My insulin? $22 from a Canadian pharmacy. Same as my U.S. one. Just shipped with a little ice pack.
And yeah, I know it’s ‘technically illegal’ - but guess what? The FDA doesn’t care as long as it’s not counterfeit.
Stop overthinking it. Your body doesn’t care where the pill came from. It just wants to work.
And if you’re scared? Check the manufacturer. If it’s Pfizer, Novo Nordisk, Teva - you’re golden.
Also - if you’re in the U.S. and you’re paying $30 for metformin? You’re being robbed.
Do better.
December 31, 2025 AT 01:21
Justin James
Think about this - the entire global generic drug supply chain is built on a single fragile assumption: that manufacturers will always prioritize profit over safety, and that regulators will always be one step behind.
And that’s not just a flaw - it’s the design. The system was never meant to be fair. It was meant to be profitable. And the only reason it still functions at all is because of the sheer volume of pills being produced - billions of them - and the fact that most people never notice the subtle differences until it’s too late.
Every time a factory in India shuts down because of a single FDA violation, it doesn’t just cause a shortage - it triggers a chain reaction across three continents. A diabetic in Ohio skips a dose. A heart patient in Germany gets a different batch. A child in Brazil gets a pill with a different coating that causes nausea. And no one connects the dots because the labels all say the same thing: ‘Metformin 500mg.’
But the fillers? The binders? The drying process? The humidity levels during packaging? The calibration of the tablet press? All different. All untracked. All invisible.
And the FDA? They inspect one factory out of every 500. They rely on reports. On paperwork. On trust.
Meanwhile, the same companies that make the pills in India also make them in Puerto Rico and China and Hungary - and the U.S. version costs 10x more. Why? Because the U.S. market is the only one that still allows price gouging after patent expiry. No other country does it this way.
And yet we act surprised when people die from ‘side effects’ that were never reported because the system doesn’t track them by batch.
This isn’t about globalization. It’s about exploitation disguised as efficiency.
We don’t need more regulations.
We need transparency.
Every pill should have a public digital fingerprint. Every batch, every factory, every test result. Open. Accessible. Live.
Until then? We’re not patients.
We’re lab rats in a global experiment with no consent form.
January 1, 2026 AT 01:57