Tag: bioequivalence
- Colin Hurd
- Feb, 4 2026
- 14 Comments
Generic vs Brand-Name Drugs: Long-Term Safety Studies Explained
Exploring long-term safety of generic vs brand-name drugs. Examines FDA regulations, real-world studies, manufacturing impacts, and expert insights on therapeutic equivalence.
- Colin Hurd
- Jan, 12 2026
- 13 Comments
FDA Safety Standards: How Generic Drugs Meet Brand Name Drug Requirements
FDA requires generic drugs to meet the same safety, strength, and bioequivalence standards as brand-name drugs. Learn how generics are tested, approved, and monitored to ensure they work just as well.
- Colin Hurd
- Jan, 1 2026
- 10 Comments
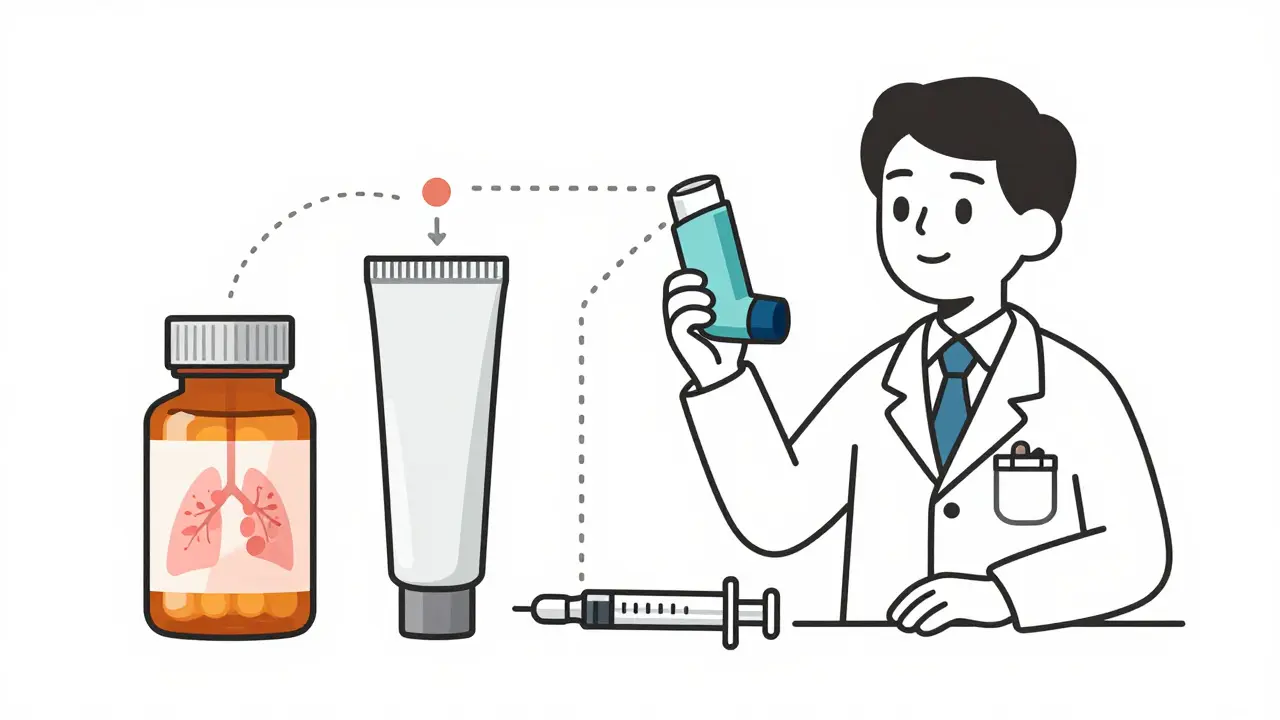
Complex Generic Formulations: Why Proving Bioequivalence Is So Hard
Proving bioequivalence for complex generic drugs-like inhalers, topical creams, and injectables-is far harder than for simple pills. Learn why these life-saving generics are still rare and what’s being done to change that.
- Colin Hurd
- Jan, 1 2026
- 14 Comments
Complex Generic Formulations: Why Proving Bioequivalence Is So Hard
Proving bioequivalence for complex generics like inhalers, creams, and injections is far harder than for regular pills. Learn why these life-saving drugs are so rare-and what’s being done to change that.
- Colin Hurd
- Dec, 19 2025
- 11 Comments
How to Compare Generic Manufacturers and Pill Appearance
Learn how to identify and compare generic drug manufacturers and pill appearances. Understand why generics look different, how to verify they're correct, and when to stay with the same manufacturer for safety.